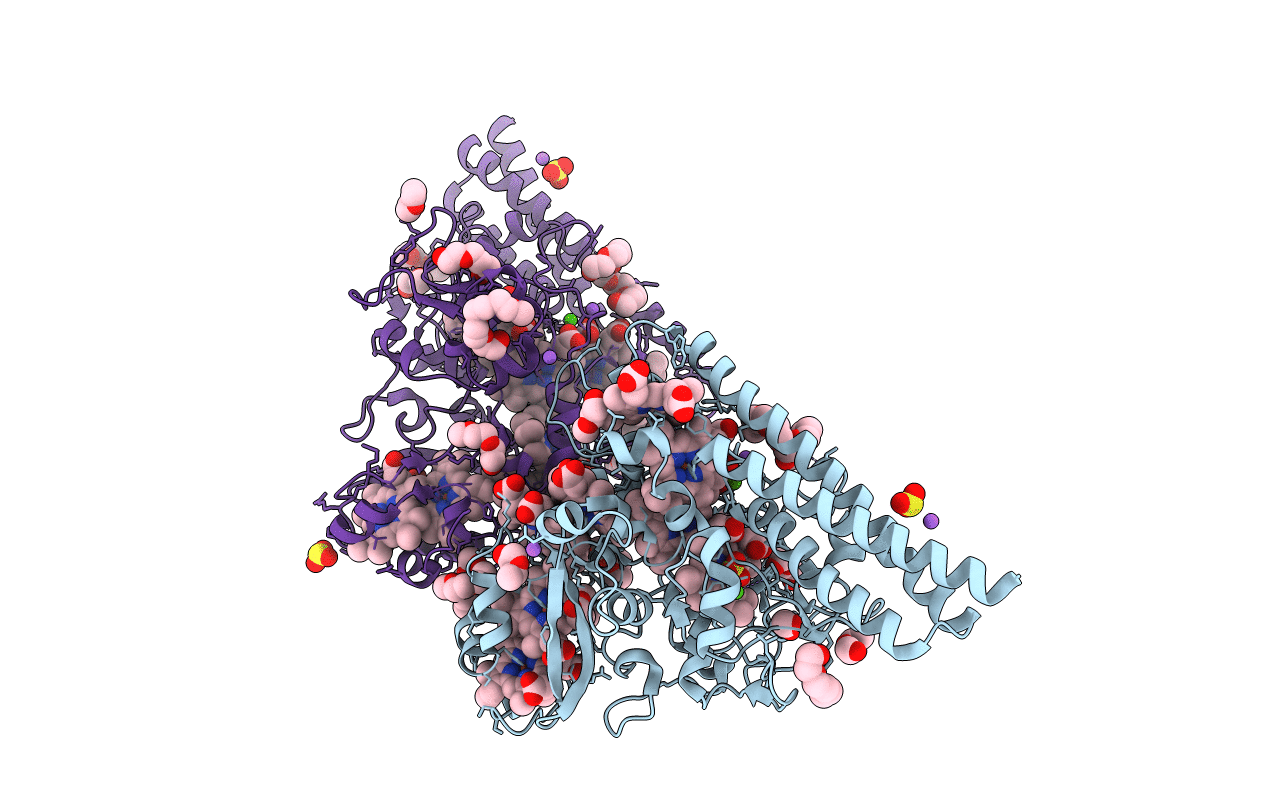
Deposition Date
2010-01-19
Release Date
2010-12-29
Last Version Date
2024-11-06
Entry Detail
PDB ID:
3LG1
Keywords:
Title:
Structure of the Thioalkalivibrio nitratireducens cytochrome c nitrite reductase reduced by sodium borohydride (in complex with sulfite)
Biological Source:
Source Organism(s):
Thioalkalivibrio nitratireducens (Taxon ID: 186931)
Method Details:
Experimental Method:
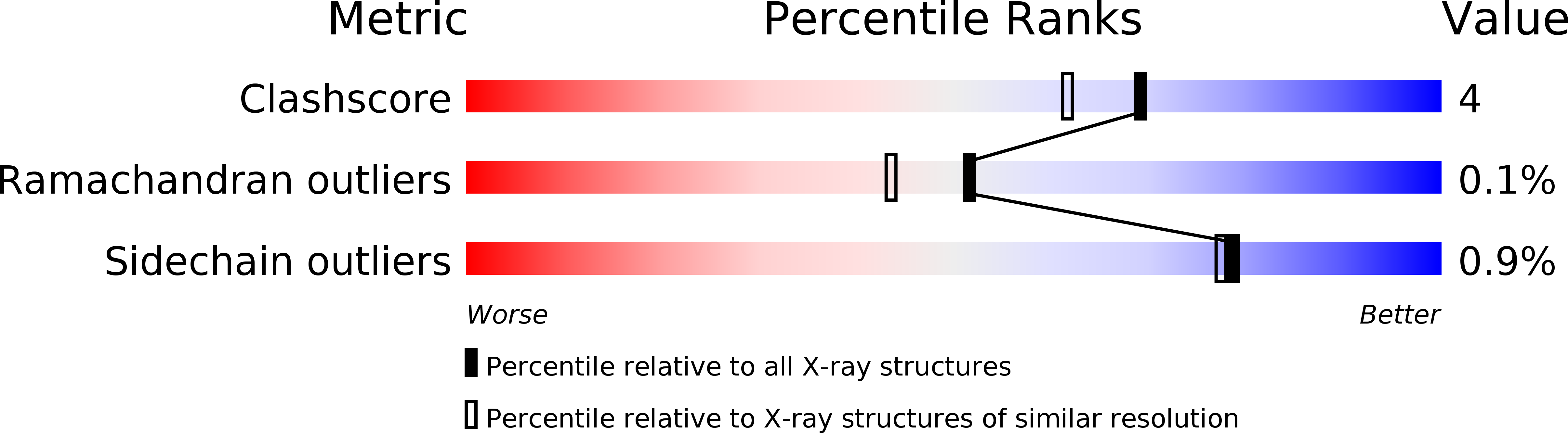
Resolution:
1.95 Å
R-Value Free:
0.18
R-Value Work:
0.16
R-Value Observed:
0.16
Space Group:
P 21 3