Deposition Date
2009-10-16
Release Date
2009-12-15
Last Version Date
2024-10-16
Entry Detail
PDB ID:
3K9X
Keywords:
Title:
X-ray crystal structure of human fxa in complex with (S)-N-((2-METHYLBENZOFURAN-5-YLAMINO)(2-OXO-1-(2-OXO-2- (PYRROLIDIN-1-YL)ETHYL)AZEPAN-3- YLAMINO)METHYLENE)NICOTINAMIDE
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Method Details:
Experimental Method:
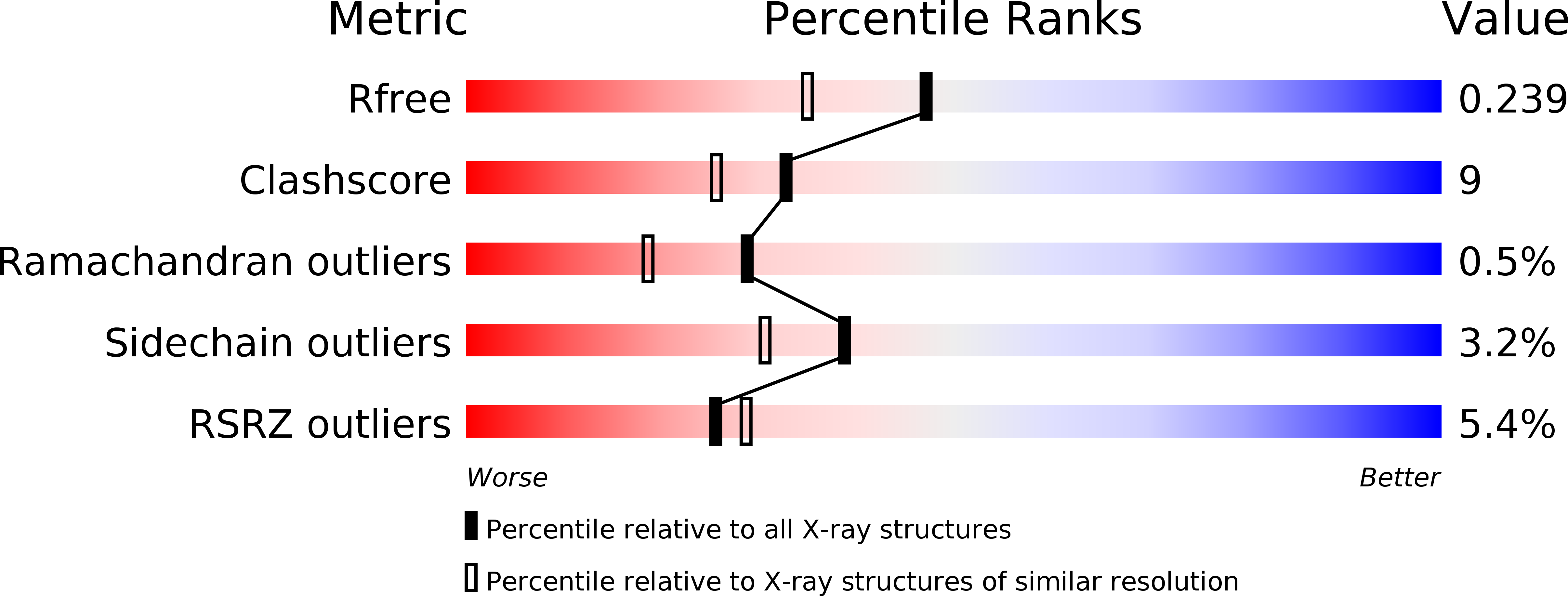
Resolution:
1.90 Å
R-Value Free:
0.24
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
P 1 21 1