Deposition Date
2009-09-18
Release Date
2010-09-08
Last Version Date
2023-11-22
Entry Detail
PDB ID:
3JWB
Keywords:
Title:
Crystal structure of L-methionine gamma-lyase from Citrobacter freundii with norleucine
Biological Source:
Source Organism(s):
Citrobacter freundii (Taxon ID: 546)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
1.63 Å
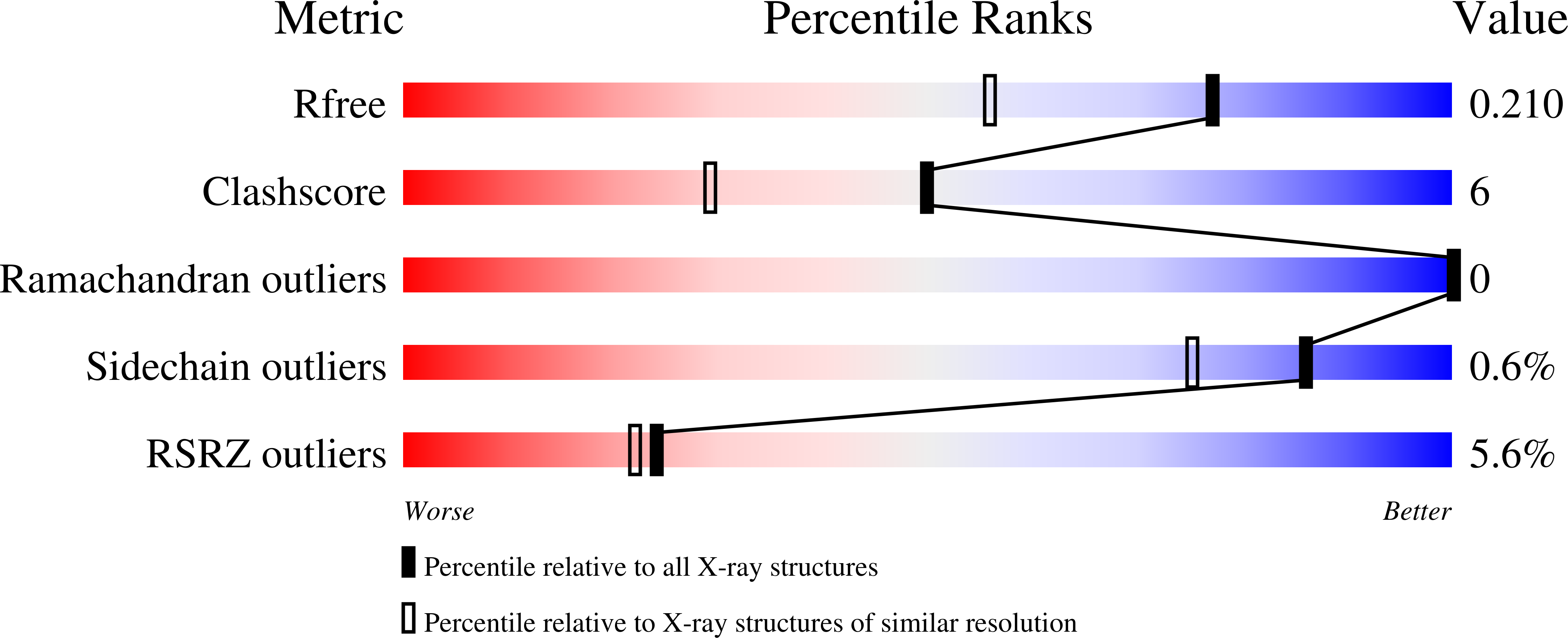
R-Value Free:
0.21
R-Value Work:
0.15
R-Value Observed:
0.16
Space Group:
I 2 2 2