Deposition Date
2009-09-11
Release Date
2011-04-06
Last Version Date
2025-03-26
Entry Detail
PDB ID:
3JTC
Keywords:
Title:
Importance of Mg2+ in the Ca2+-Dependent Folding of the gamma-Carboxyglutamic Acid Domains of Vitamin K-Dependent clotting and anticlotting Proteins
Biological Source:
Source Organism(s):
HOMO SAPIENS (Taxon ID: 9606)
Method Details:
Experimental Method:
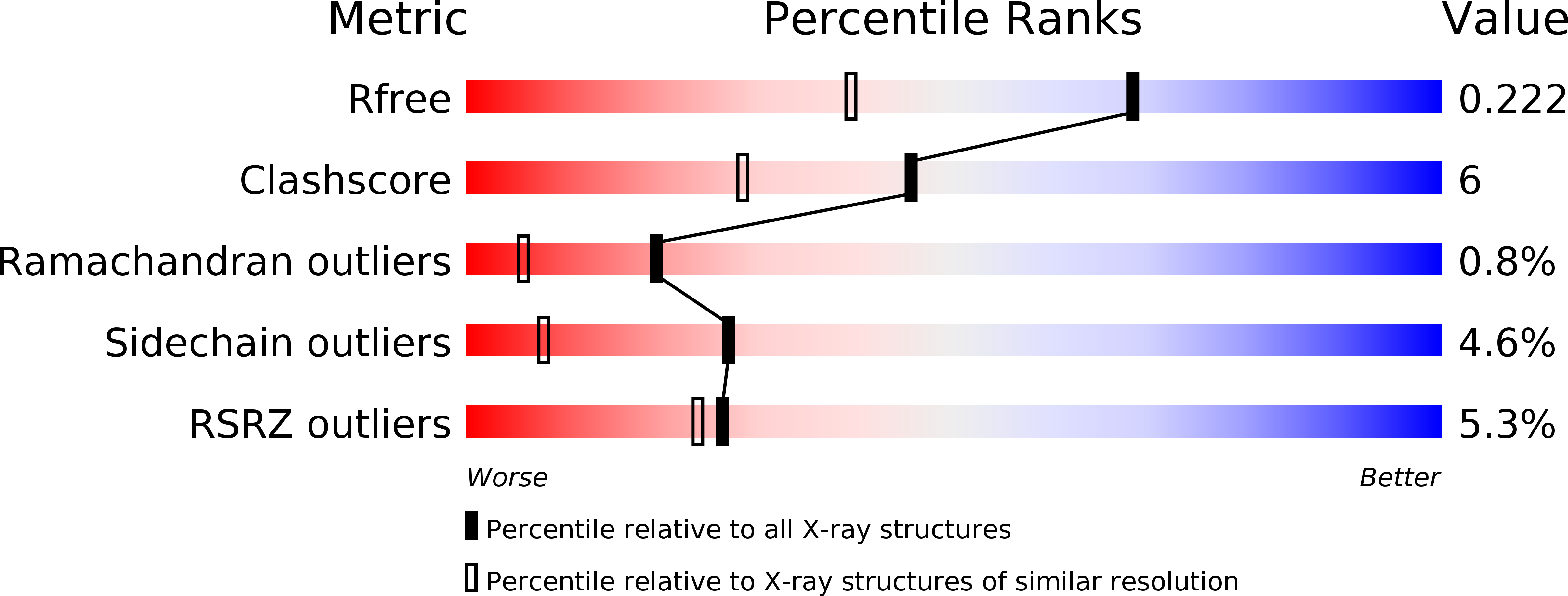
Resolution:
1.60 Å
R-Value Free:
0.22
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 1 21 1