Deposition Date
2009-09-07
Release Date
2009-12-01
Last Version Date
2024-10-16
Entry Detail
PDB ID:
3JQO
Keywords:
Title:
Crystal structure of the outer membrane complex of a type IV secretion system
Biological Source:
Source Organism:
Escherichia coli (Taxon ID: 83333)
Host Organism:
Method Details:
Experimental Method:
Resolution:
2.60 Å
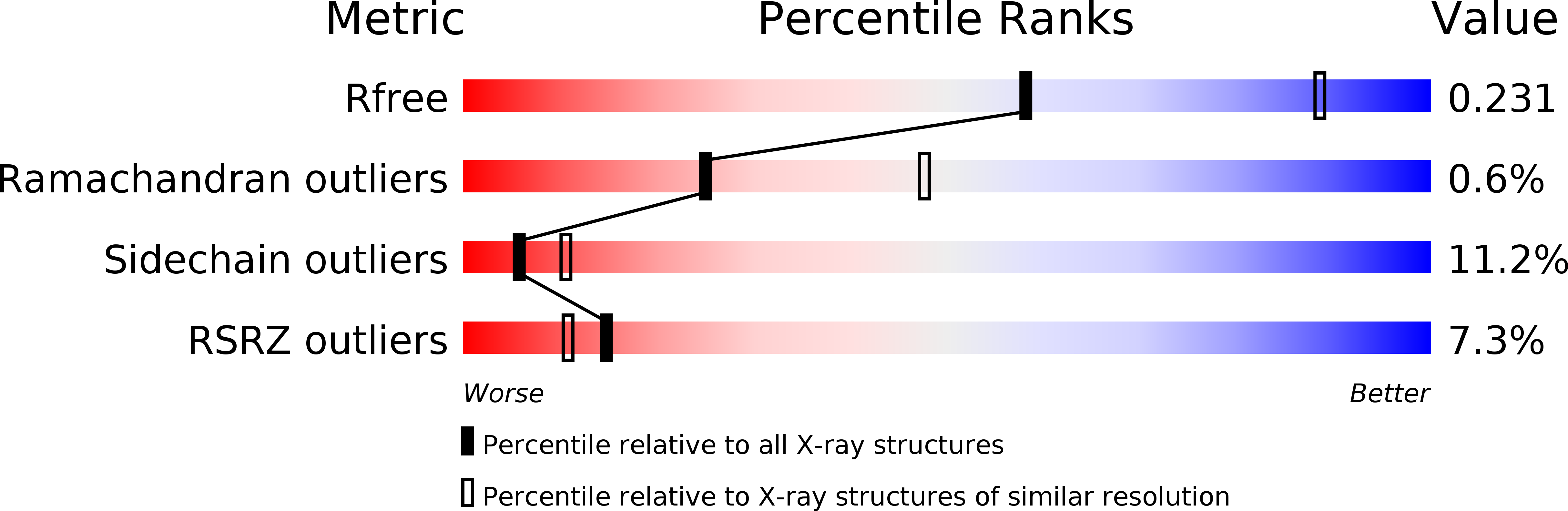
R-Value Free:
0.25
R-Value Work:
0.22
R-Value Observed:
0.22
Space Group:
P 21 21 2