Deposition Date
2015-12-11
Release Date
2016-02-17
Last Version Date
2024-02-21
Entry Detail
PDB ID:
3JCG
Keywords:
Title:
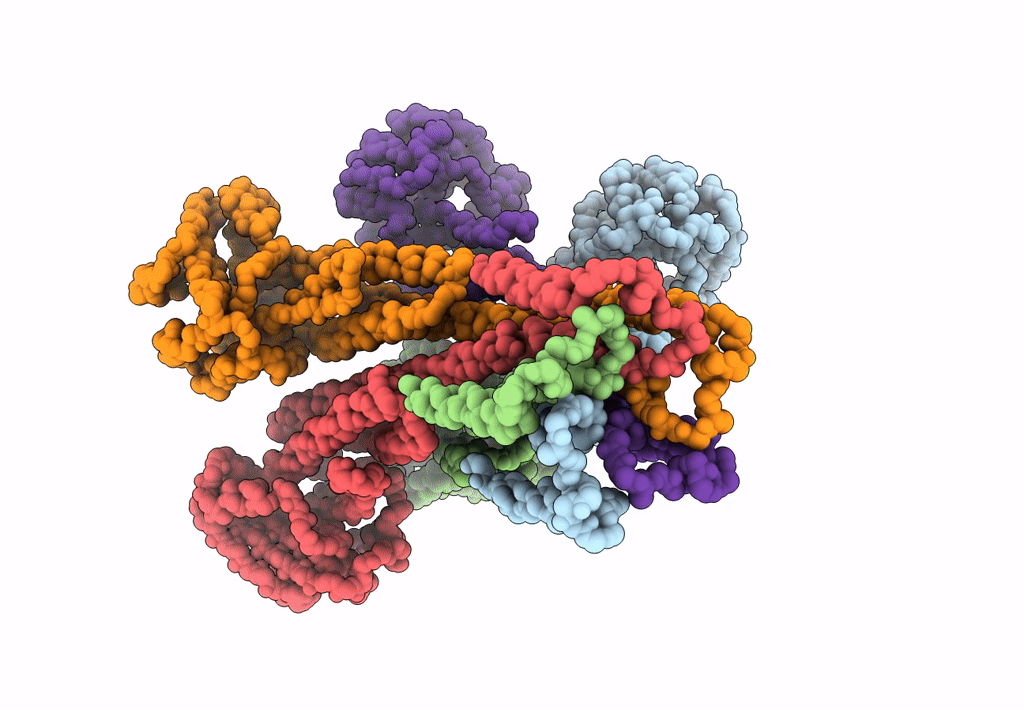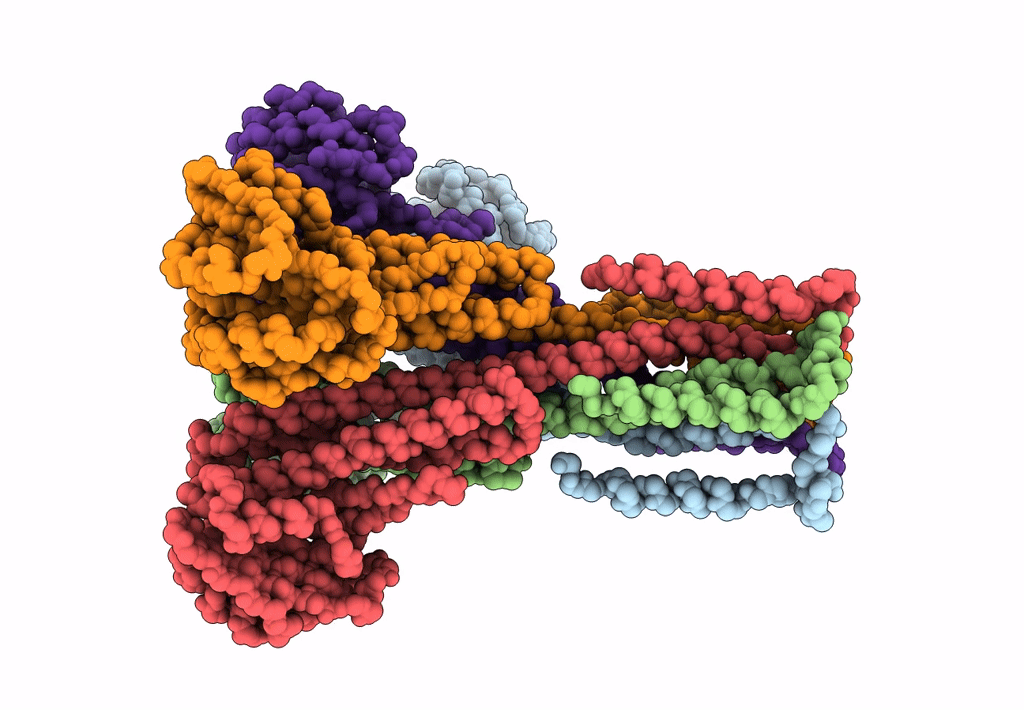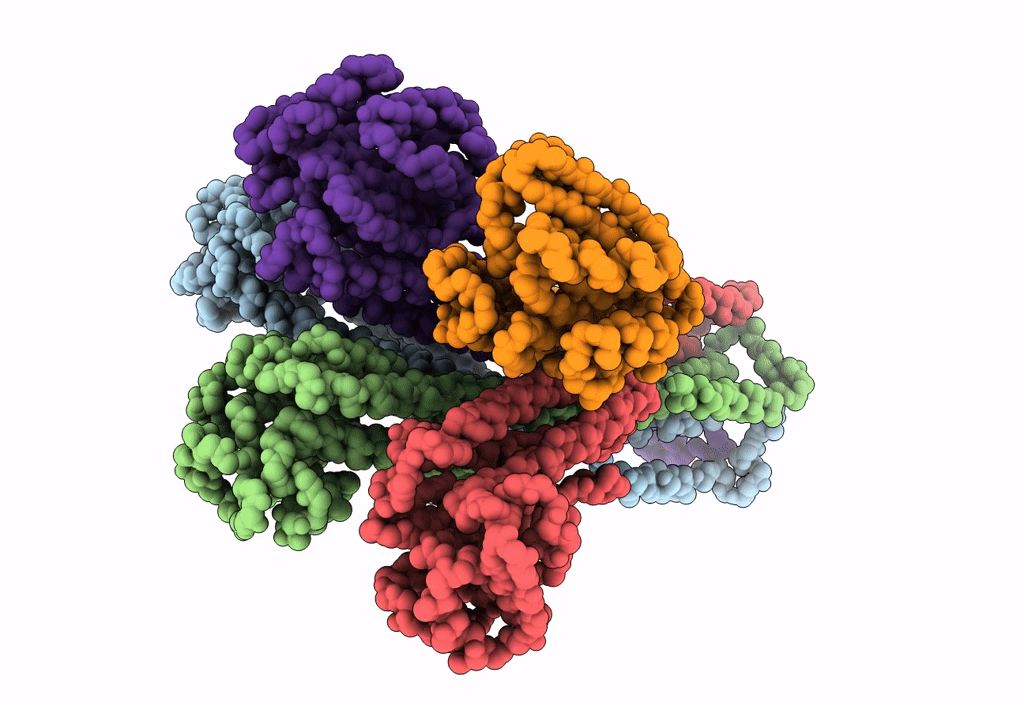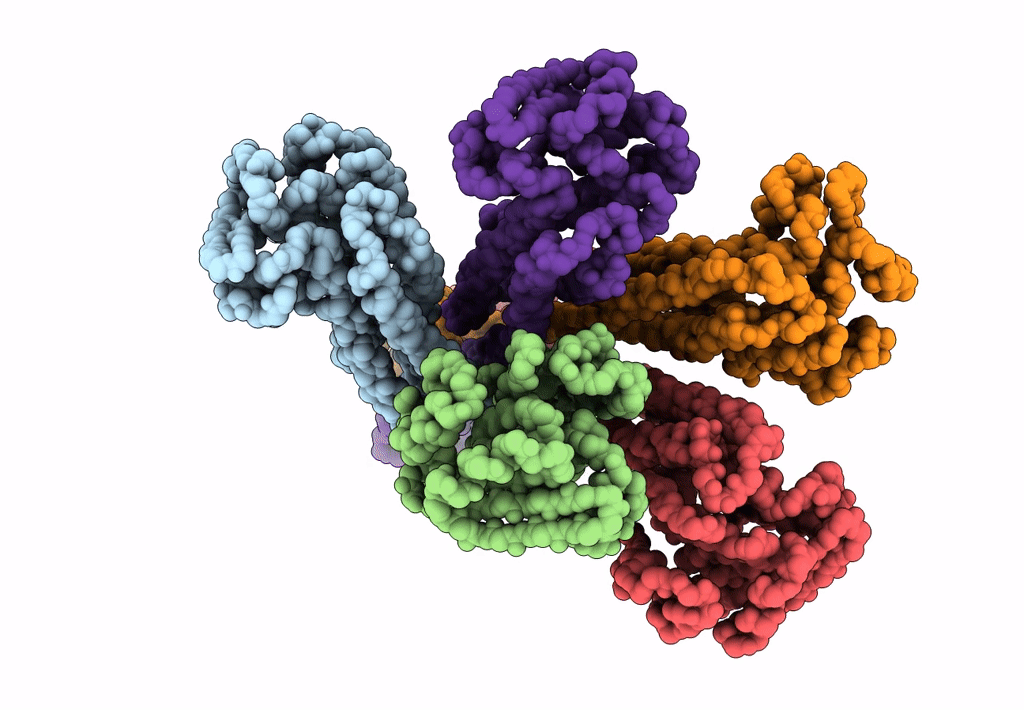
Cryo-EM structure of the magnesium channel CorA in the magnesium-free, asymmetric open state I
Biological Source:
Source Organism(s):
Thermotoga maritima (Taxon ID: 2336)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
7.06 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE


