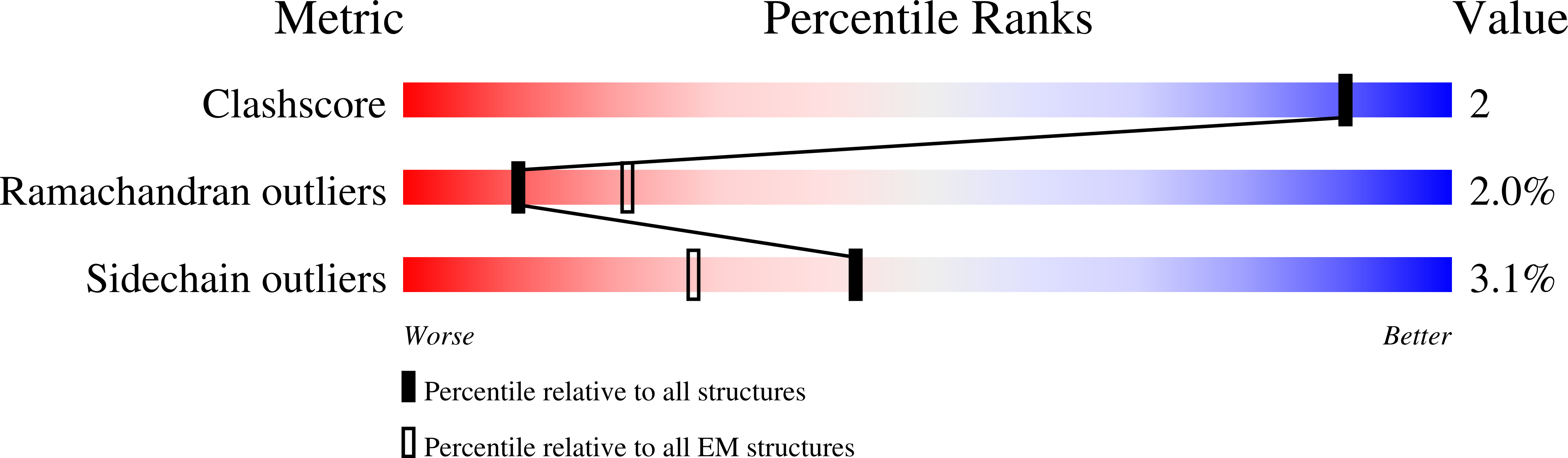
Deposition Date
2014-11-20
Release Date
2014-12-10
Last Version Date
2024-02-21
Entry Detail
PDB ID:
3J8X
Keywords:
Title:
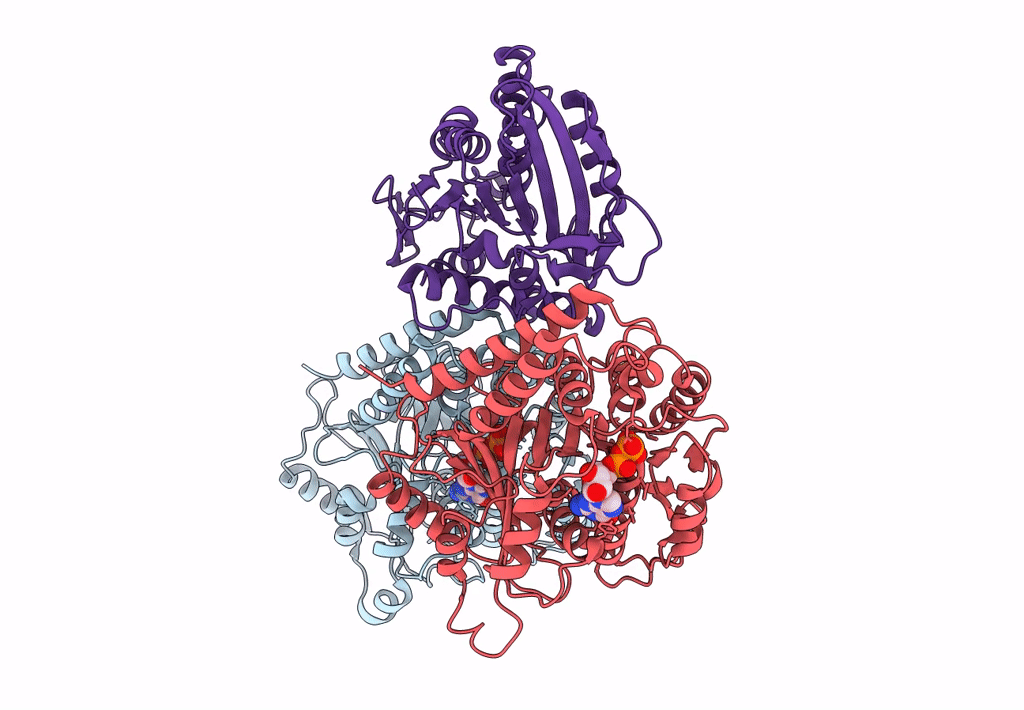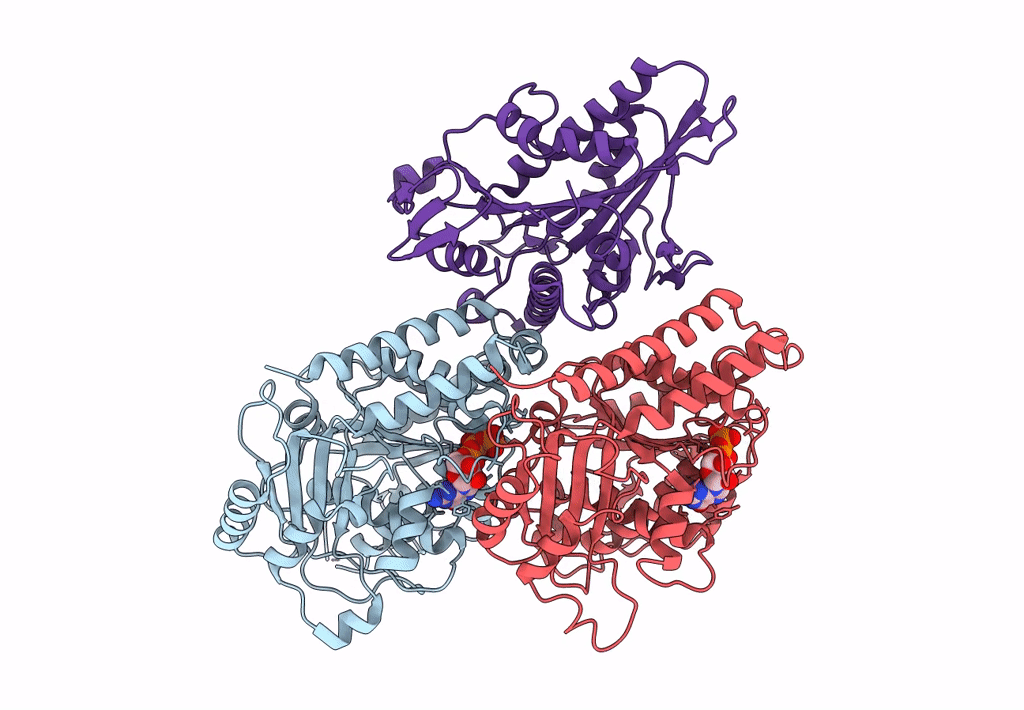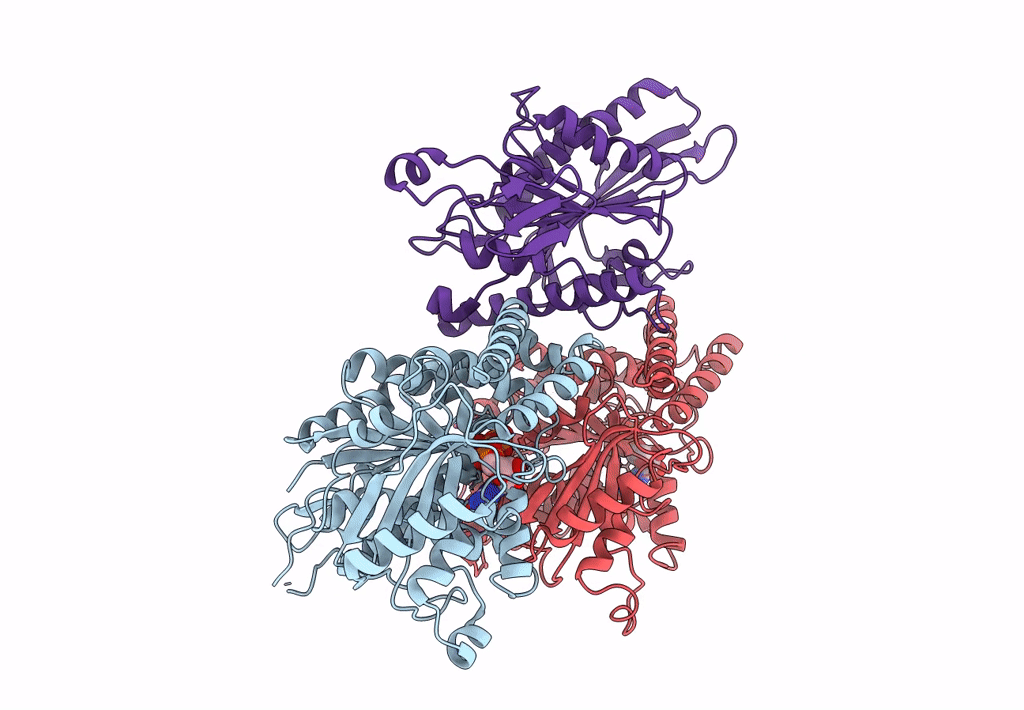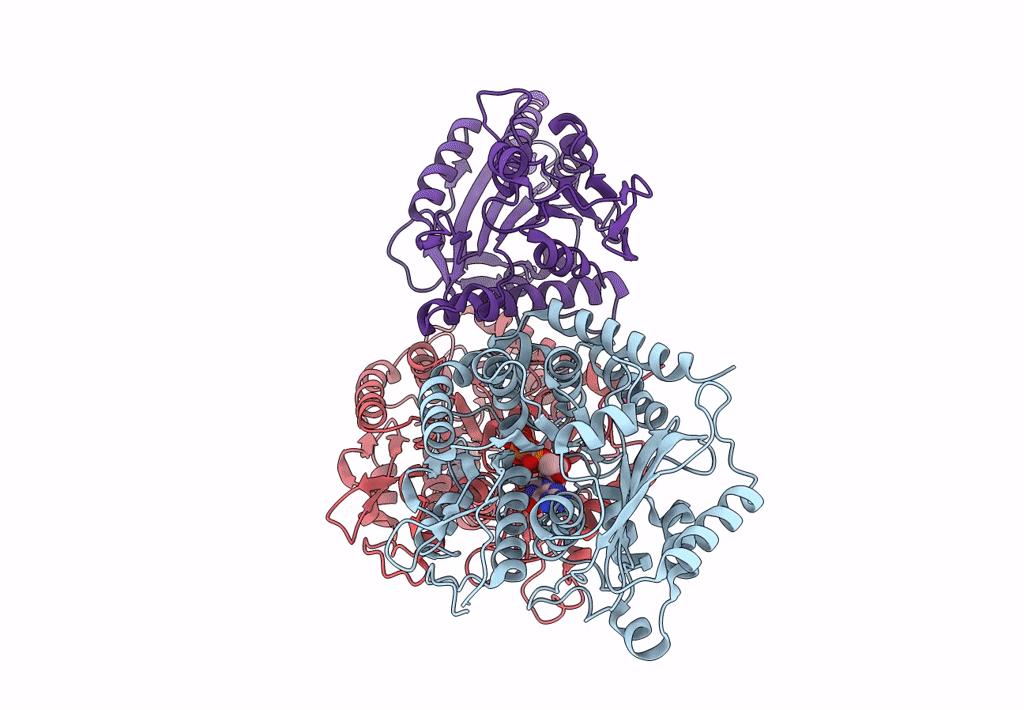
High-resolution structure of no-nucleotide kinesin on microtubules
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Sus scrofa (Taxon ID: 9823)
Sus scrofa (Taxon ID: 9823)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
5.00 Å
Aggregation State:
FILAMENT
Reconstruction Method:
HELICAL