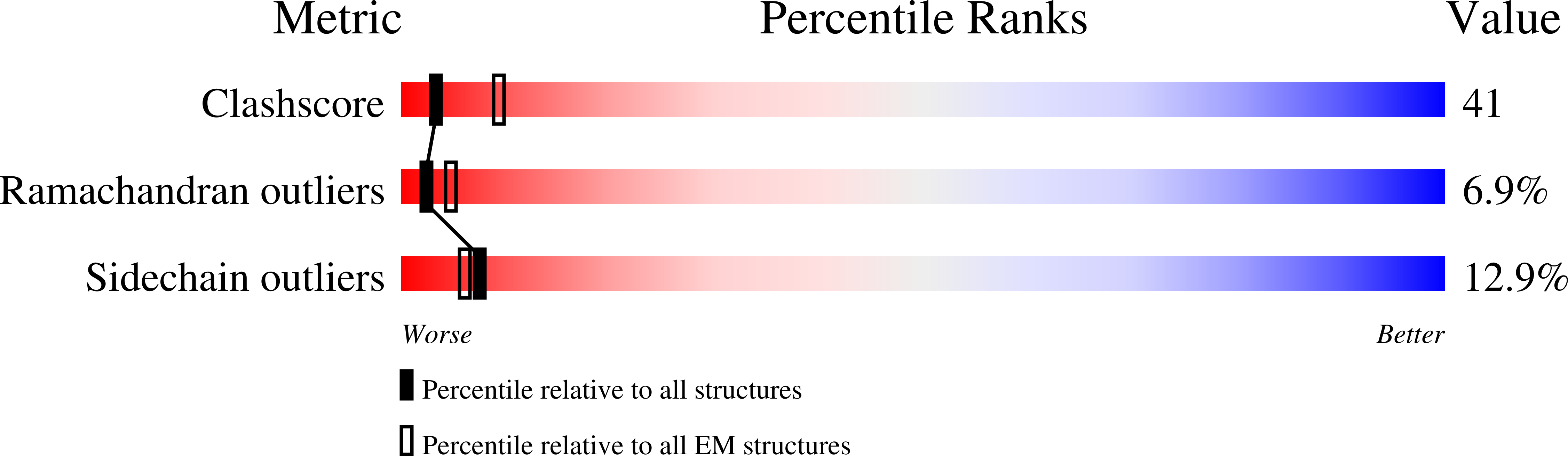
Deposition Date
2013-08-26
Release Date
2013-09-25
Last Version Date
2024-02-21
Entry Detail
PDB ID:
3J4J
Keywords:
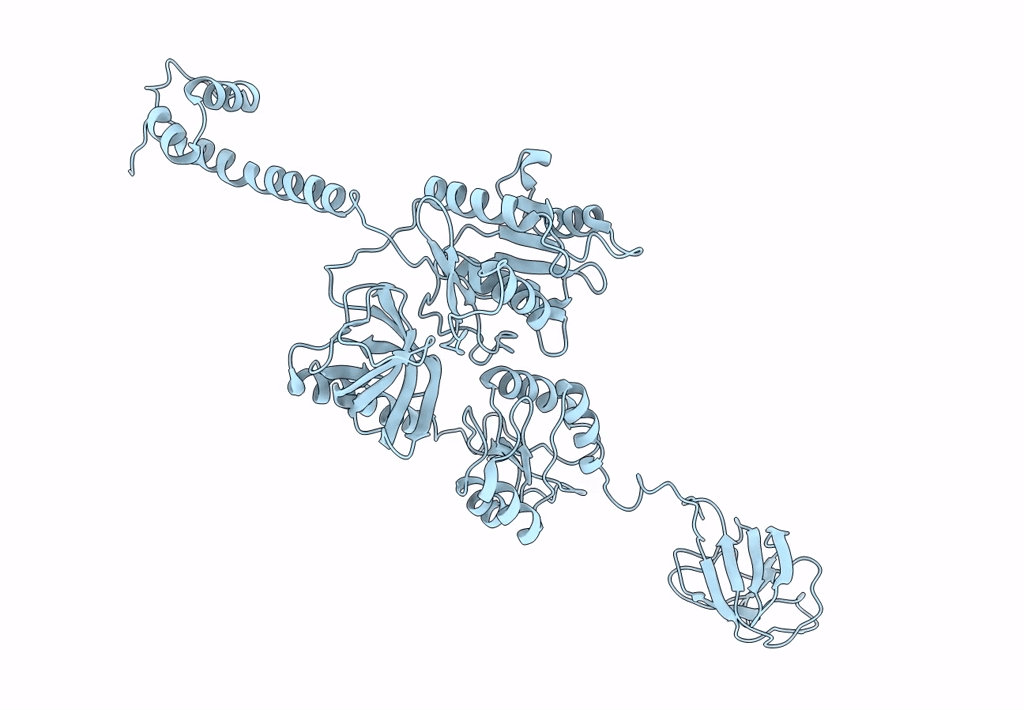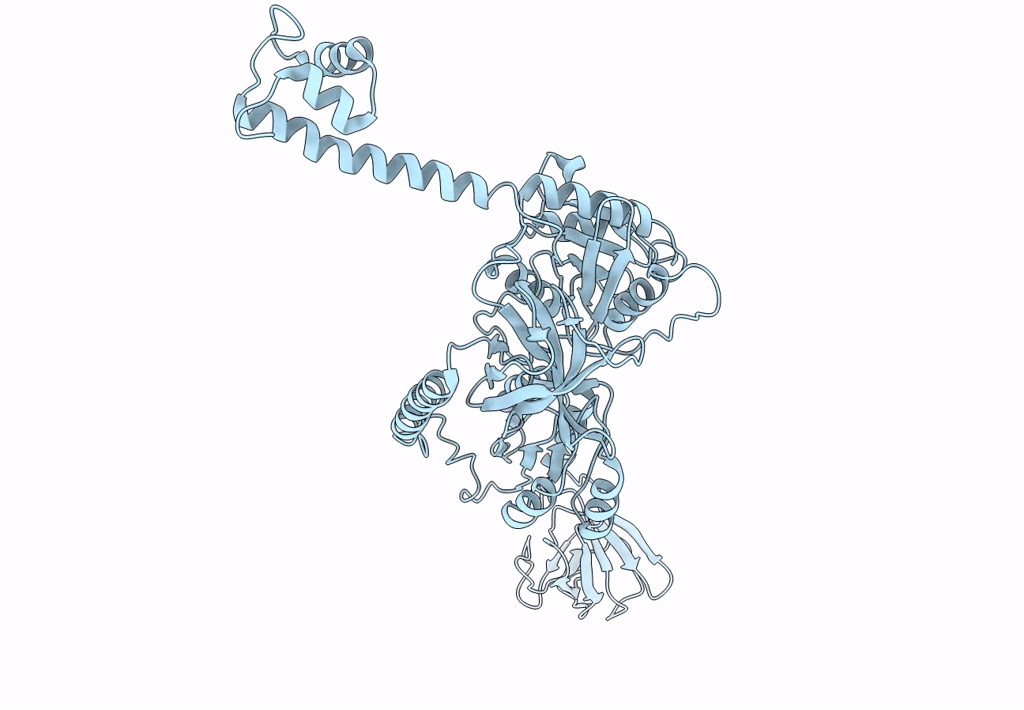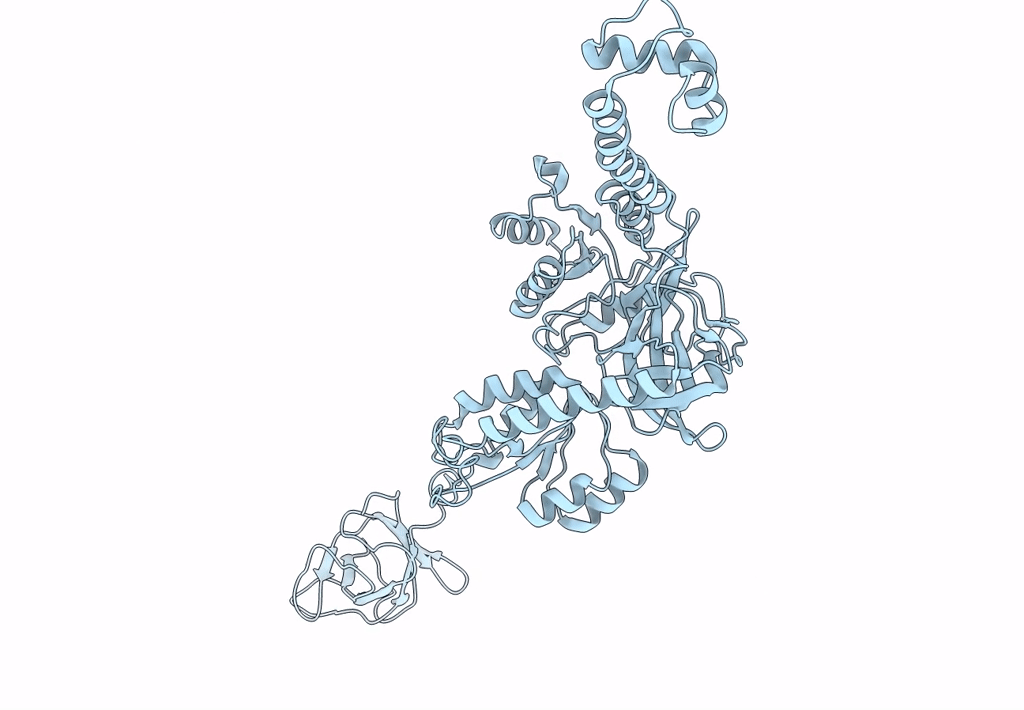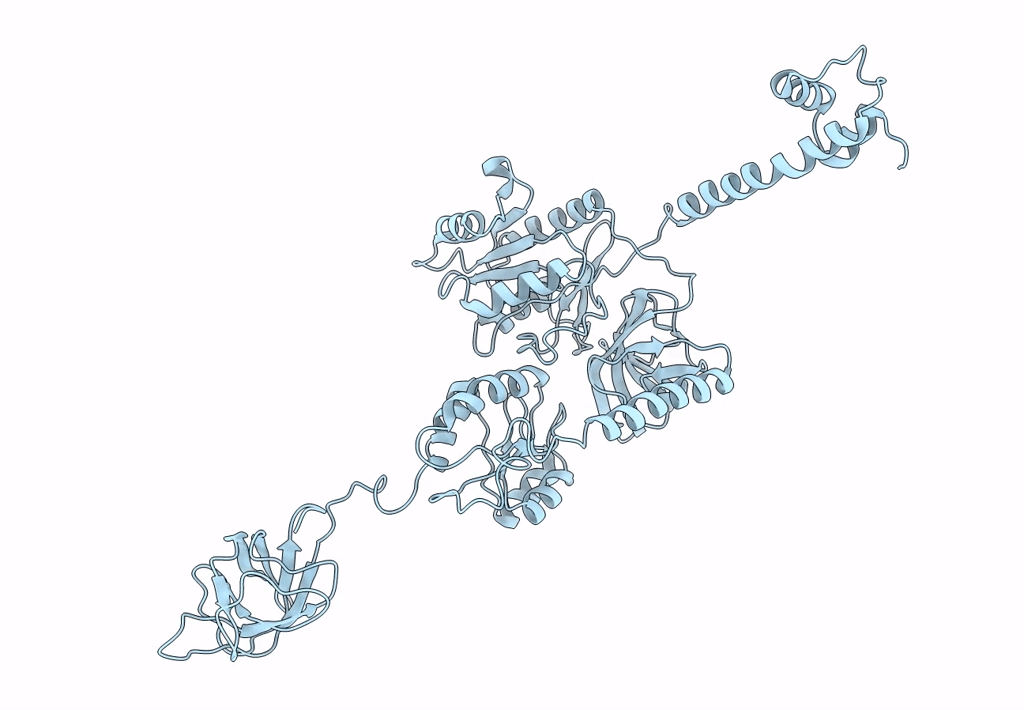
Title:
Model of full-length T. thermophilus Translation Initiation Factor 2 refined against its cryo-EM density from a 30S Initiation Complex map
Biological Source:
Source Organism(s):
Thermus thermophilus (Taxon ID: 300852)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
11.50 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE