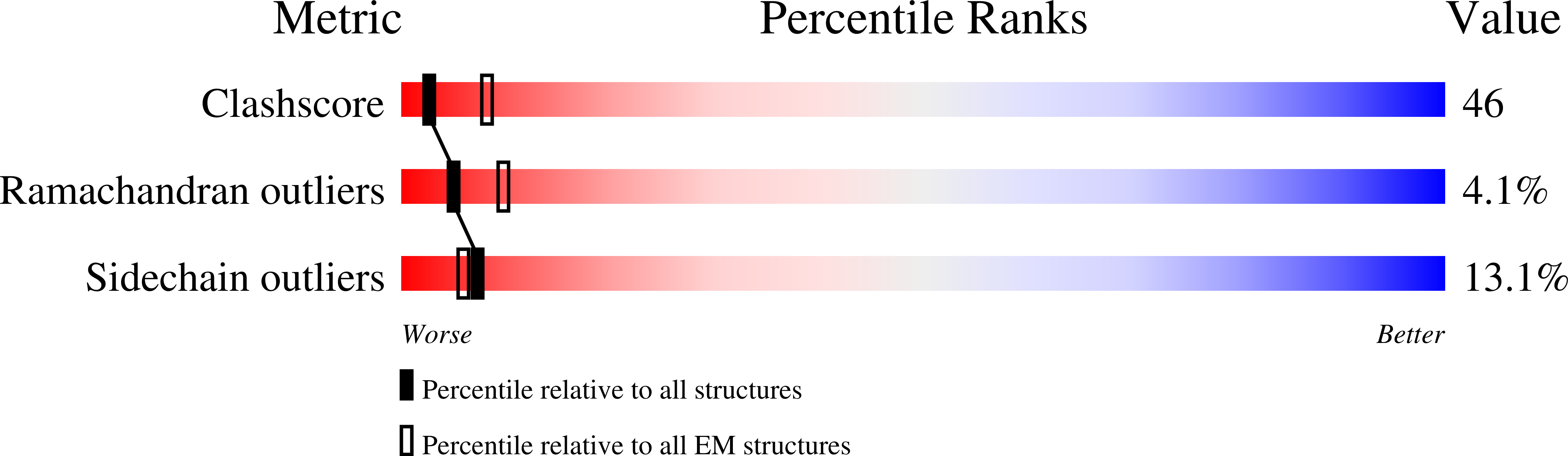
Deposition Date
2012-05-23
Release Date
2012-06-06
Last Version Date
2024-10-30
Entry Detail
PDB ID:
3J1S
Keywords:
Title:
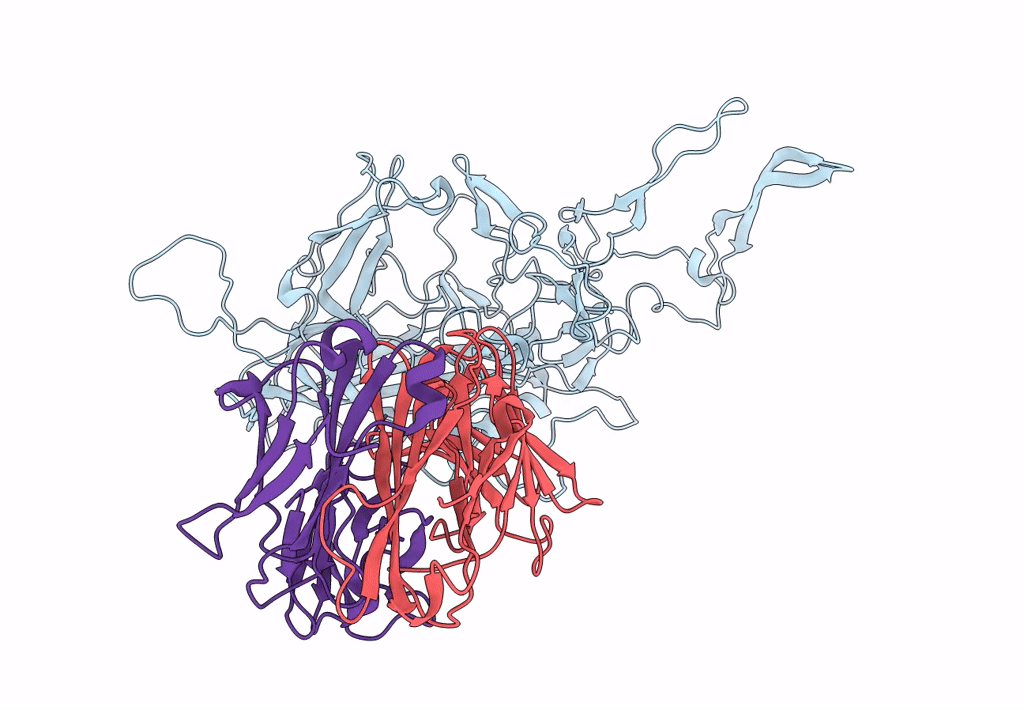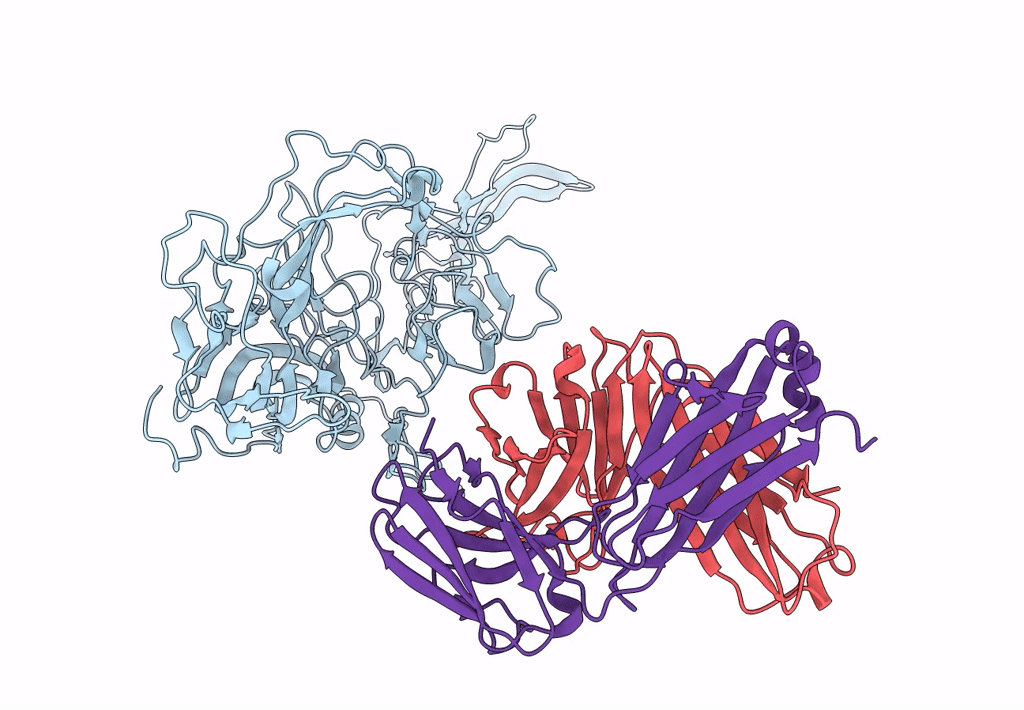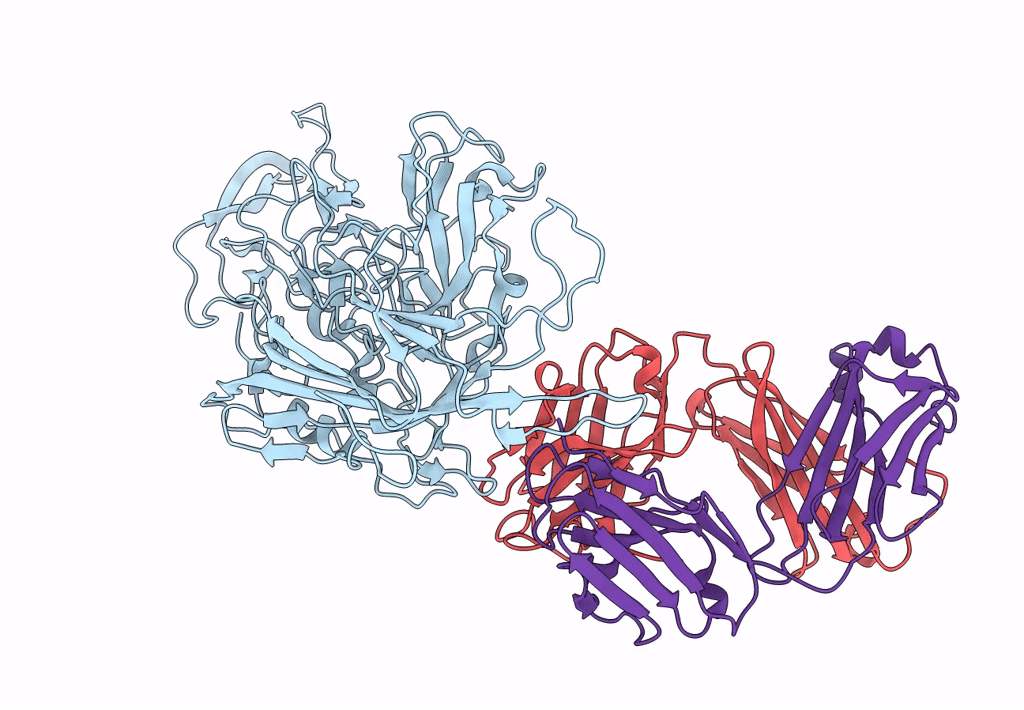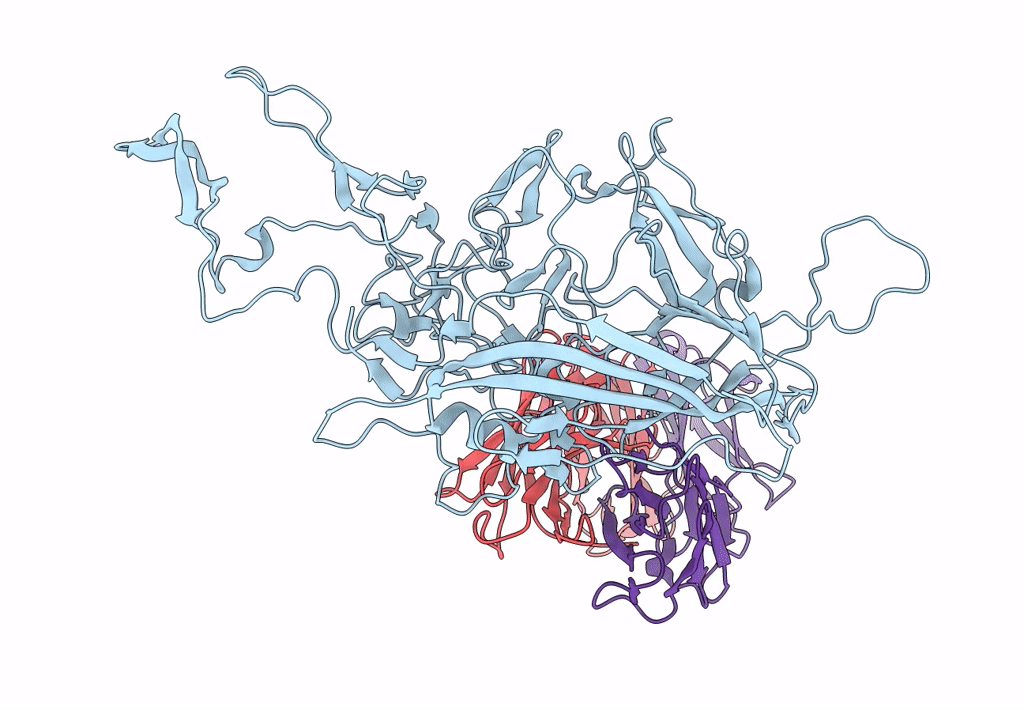
Structure of adeno-associated virus-2 in complex with neutralizing monoclonal antibody A20
Biological Source:
Source Organism(s):
Mus musculus (Taxon ID: 10090)
Adeno-associated virus - 2 (Taxon ID: 10804)
Adeno-associated virus - 2 (Taxon ID: 10804)
Method Details:
Experimental Method:
Resolution:
8.50 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE