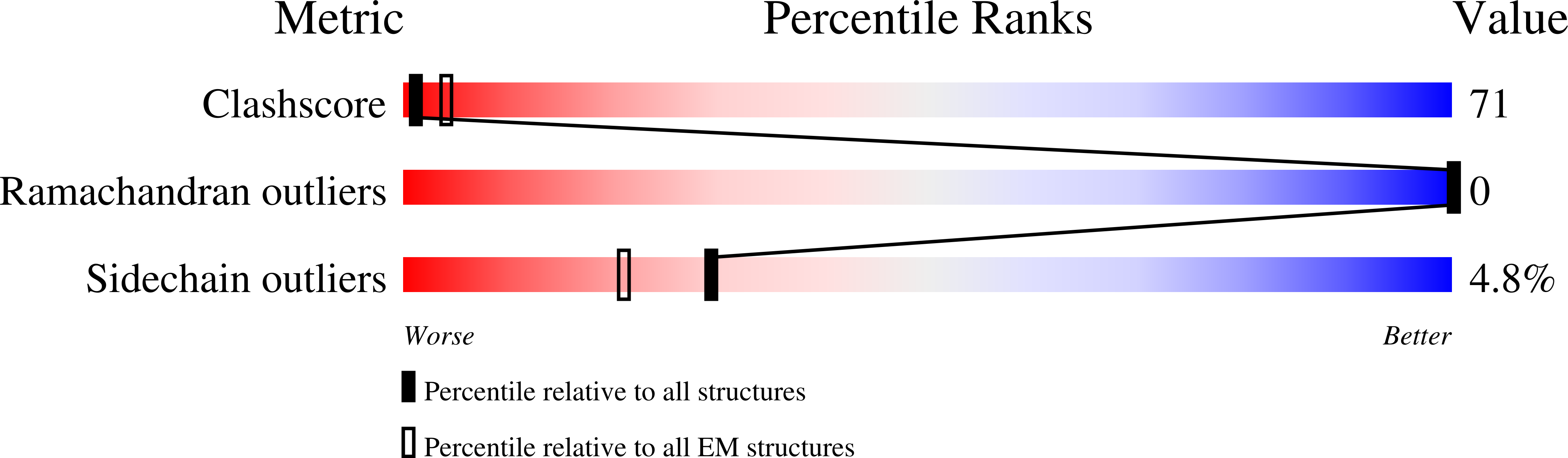
Deposition Date
2012-05-18
Release Date
2012-06-20
Last Version Date
2024-02-21
Entry Detail
PDB ID:
3J1R
Keywords:
Title:
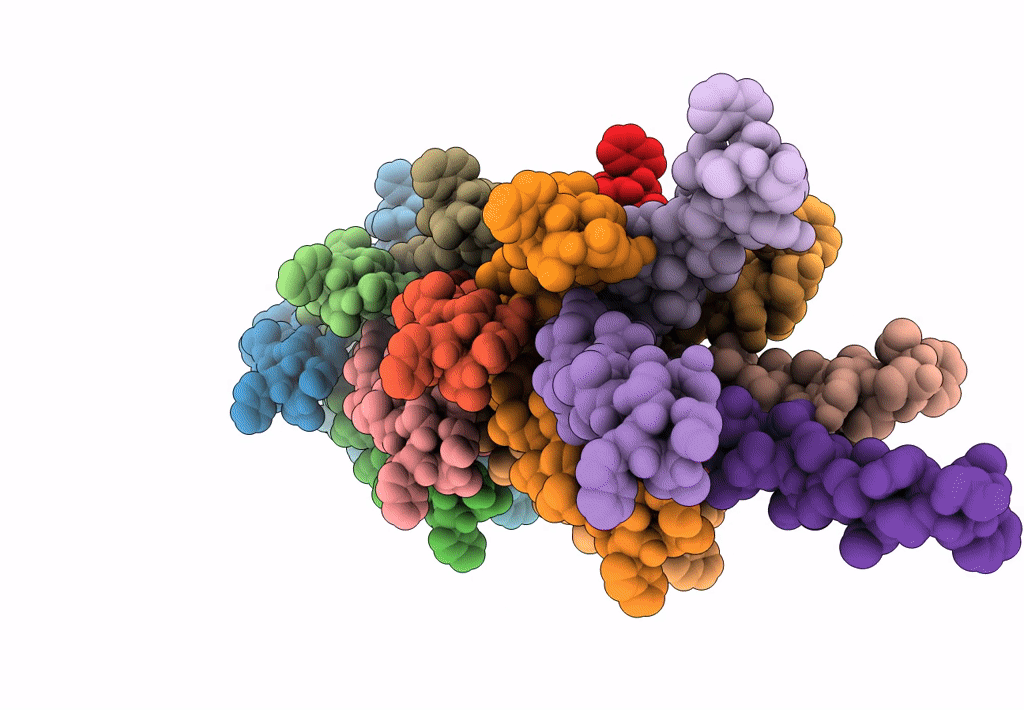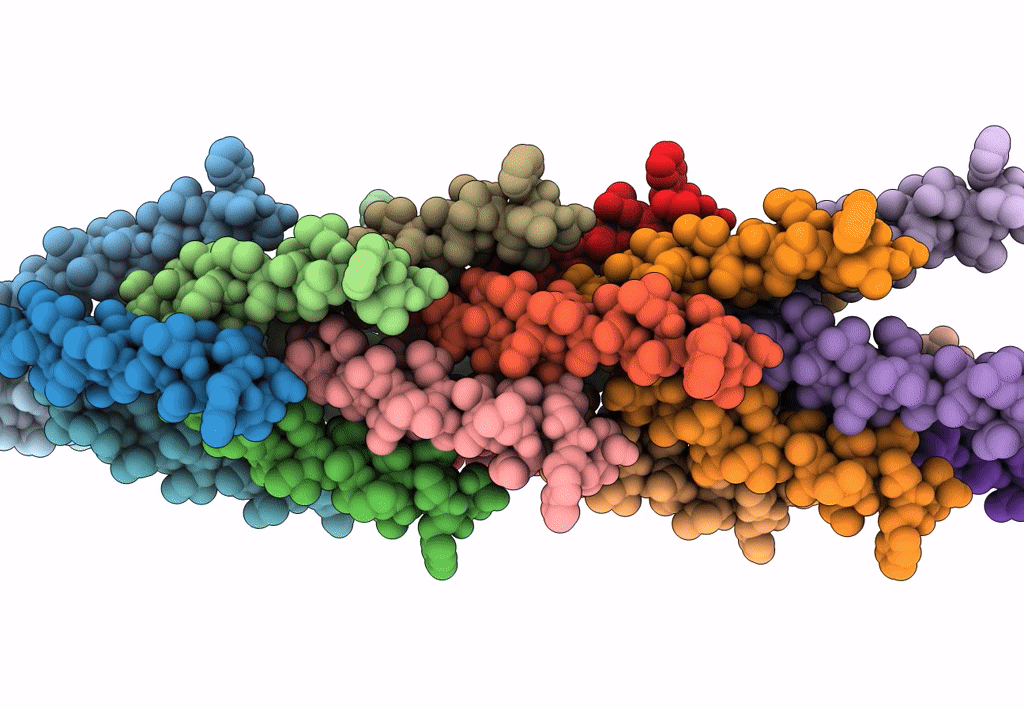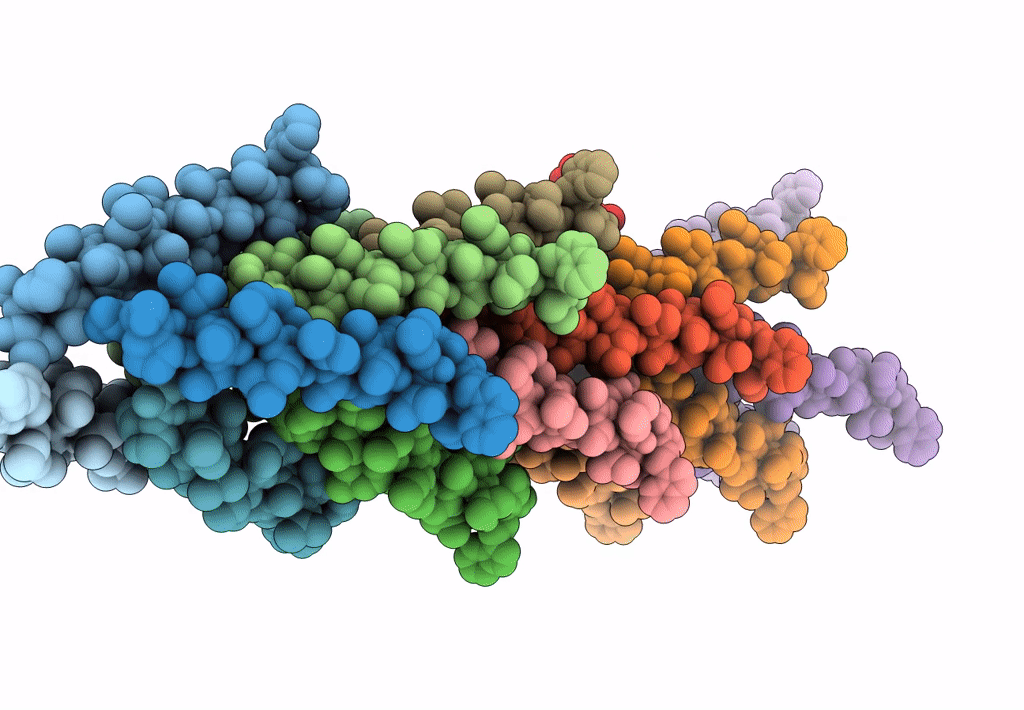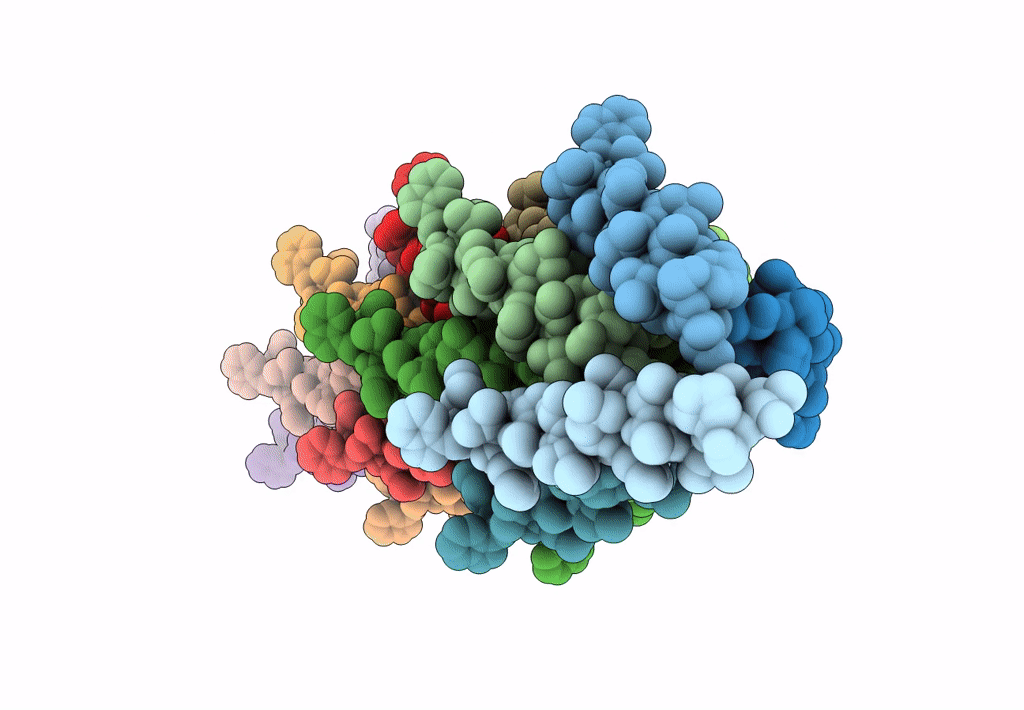
Filaments from Ignicoccus hospitalis Show Diversity of Packing in Proteins Containing N-terminal Type IV Pilin Helices
Biological Source:
Source Organism(s):
Ignicoccus hospitalis (Taxon ID: 160233)
Method Details:
Experimental Method:
Resolution:
7.50 Å
Aggregation State:
FILAMENT
Reconstruction Method:
HELICAL