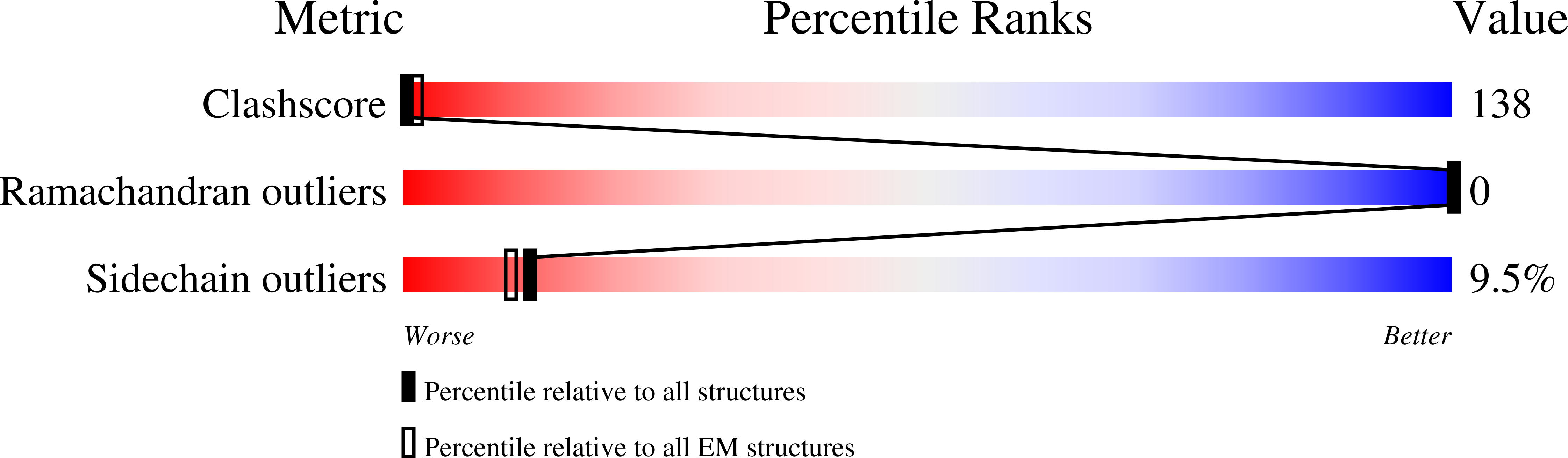
Deposition Date
2011-11-03
Release Date
2012-02-29
Last Version Date
2024-02-21
Entry Detail
PDB ID:
3J0R
Keywords:
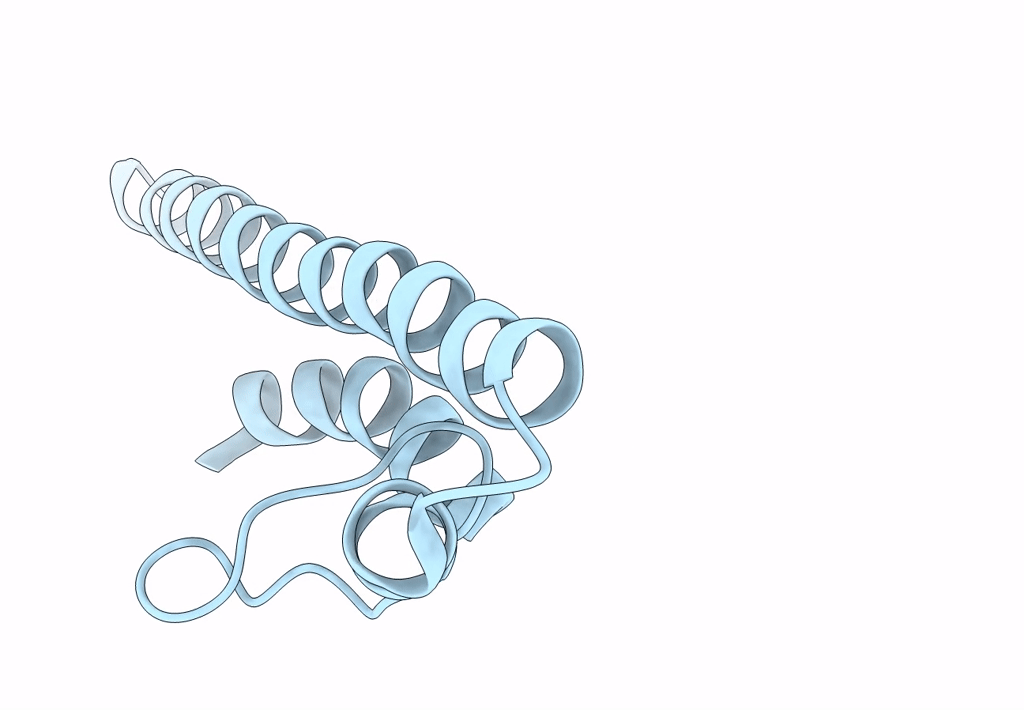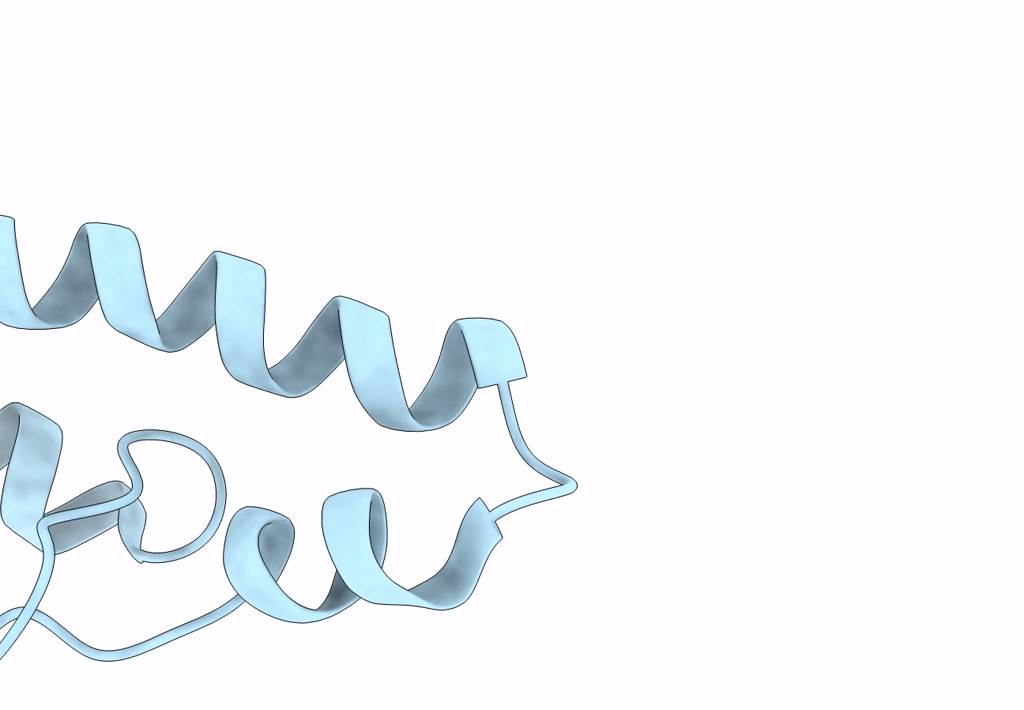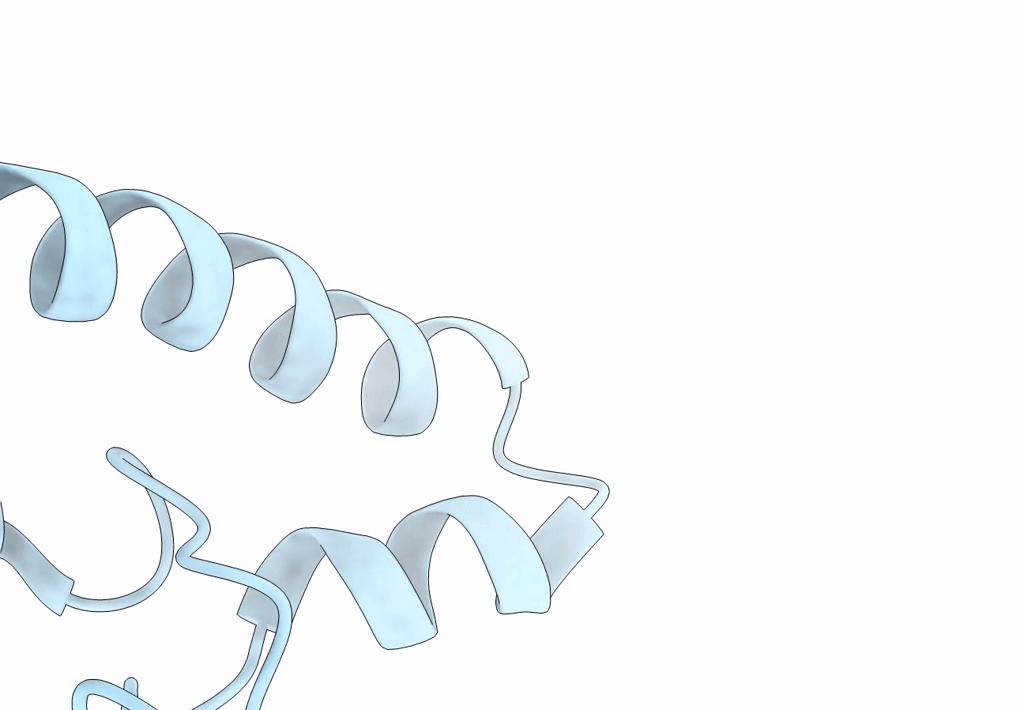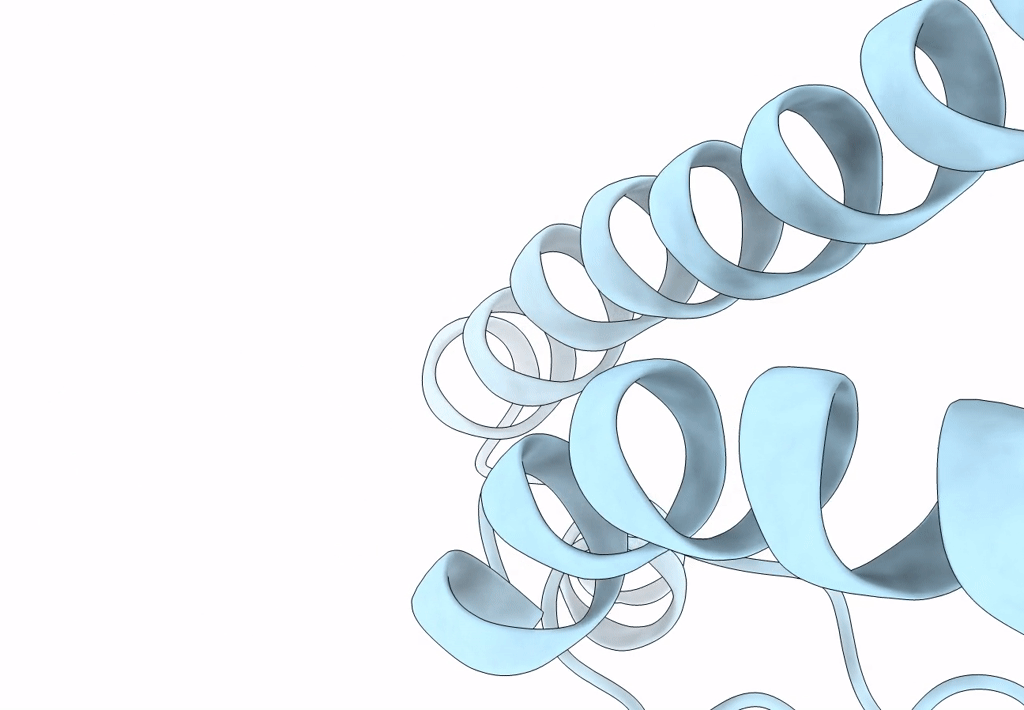
Title:
Model of a type III secretion system needle based on a 7 Angstrom resolution cryoEM map
Biological Source:
Source Organism(s):
Shigella flexneri (Taxon ID: 623)
Method Details:
Experimental Method:
Resolution:
7.70 Å
Aggregation State:
FILAMENT
Reconstruction Method:
HELICAL