Deposition Date
2011-07-21
Release Date
2011-08-24
Last Version Date
2024-11-27
Entry Detail
PDB ID:
3J0G
Keywords:
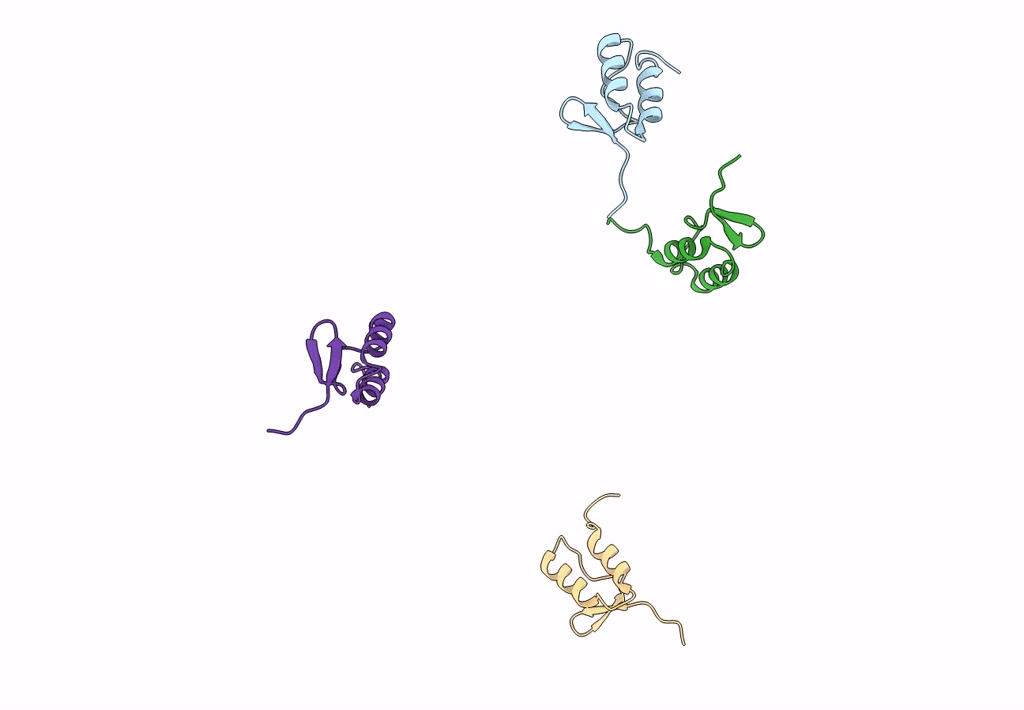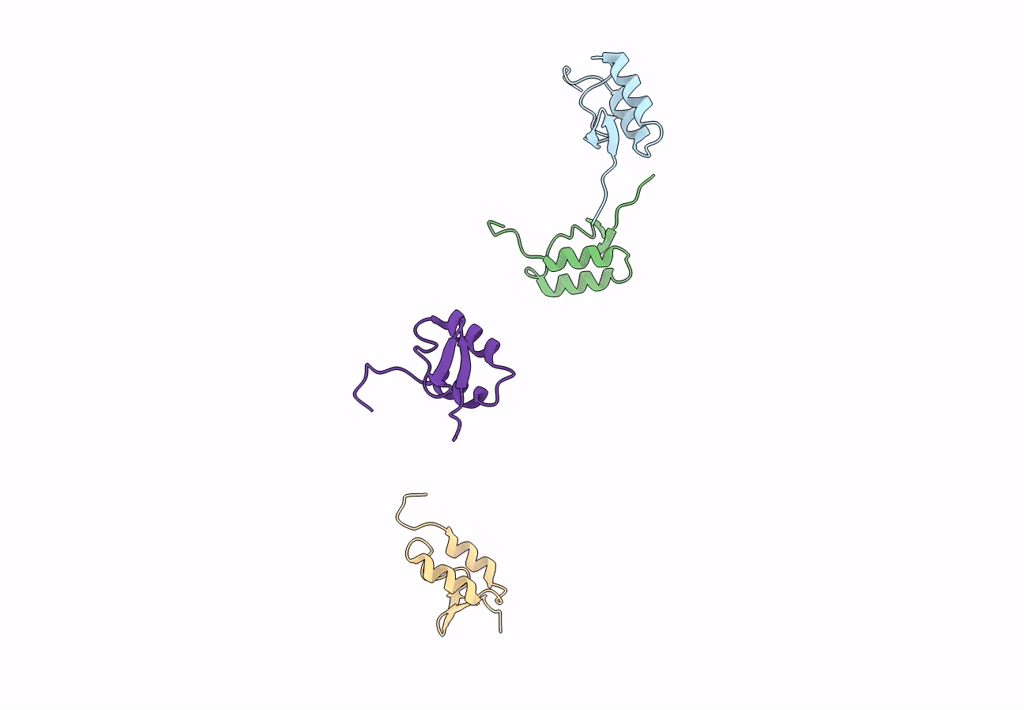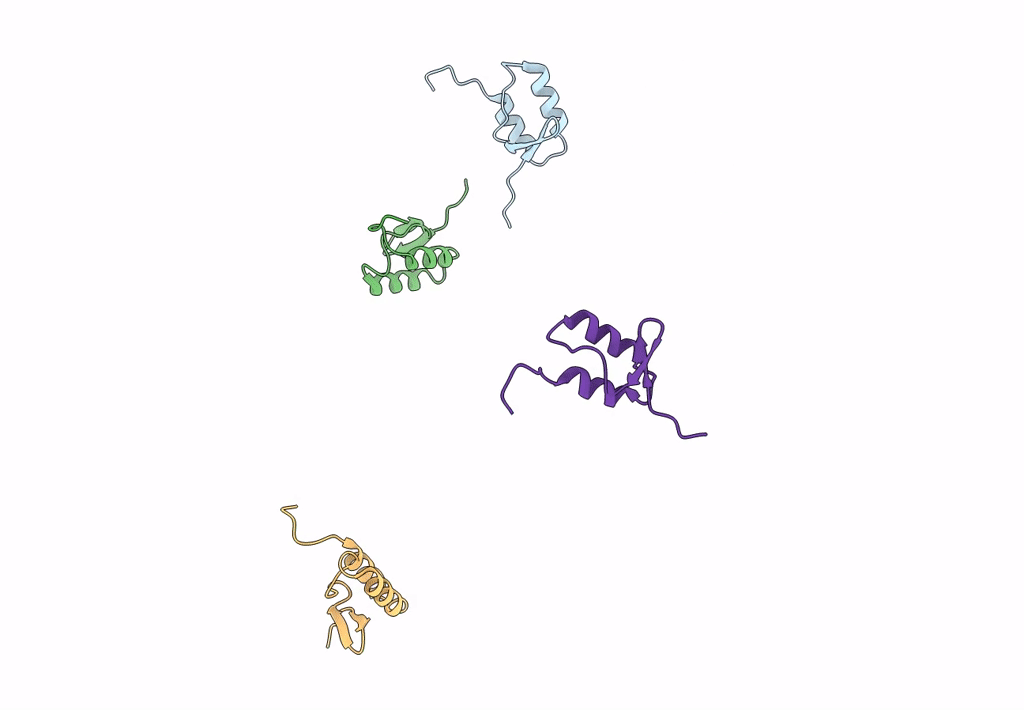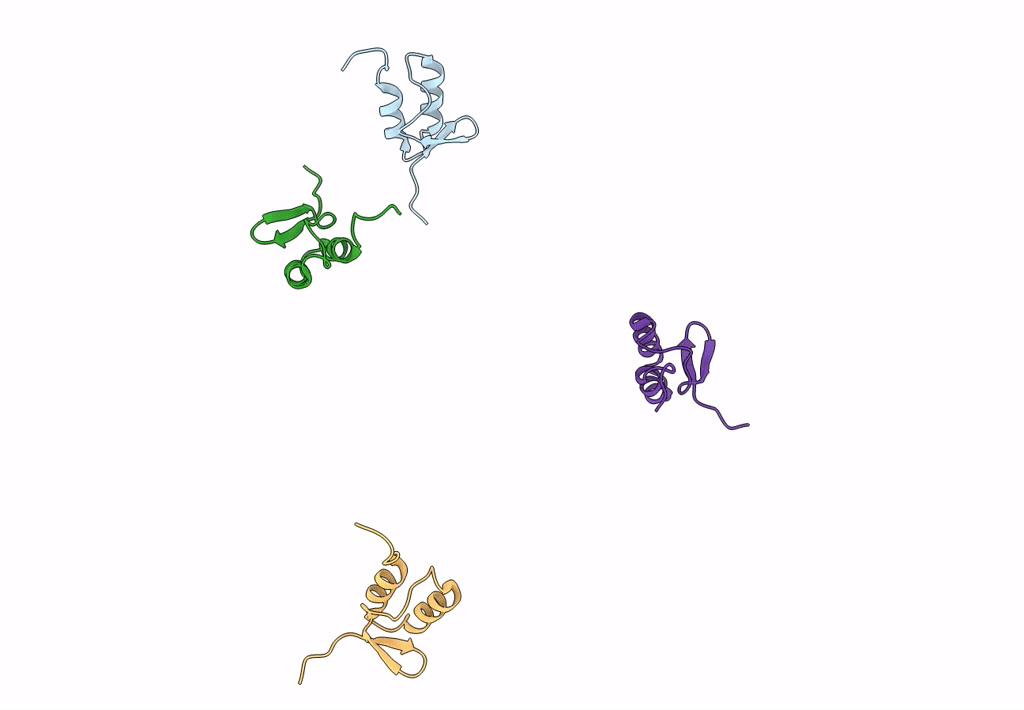
Title:
Homology model of E3 protein of Venezuelan Equine Encephalitis Virus TC-83 strain fitted with a cryo-EM map
Biological Source:
Source Organism(s):
Venezuelan equine encephalitis virus (Taxon ID: 11037)
Method Details:
Experimental Method:
Resolution:
4.80 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE


