Deposition Date
2011-01-20
Release Date
2011-03-30
Last Version Date
2024-02-21
Entry Detail
PDB ID:
3IZZ
Keywords:
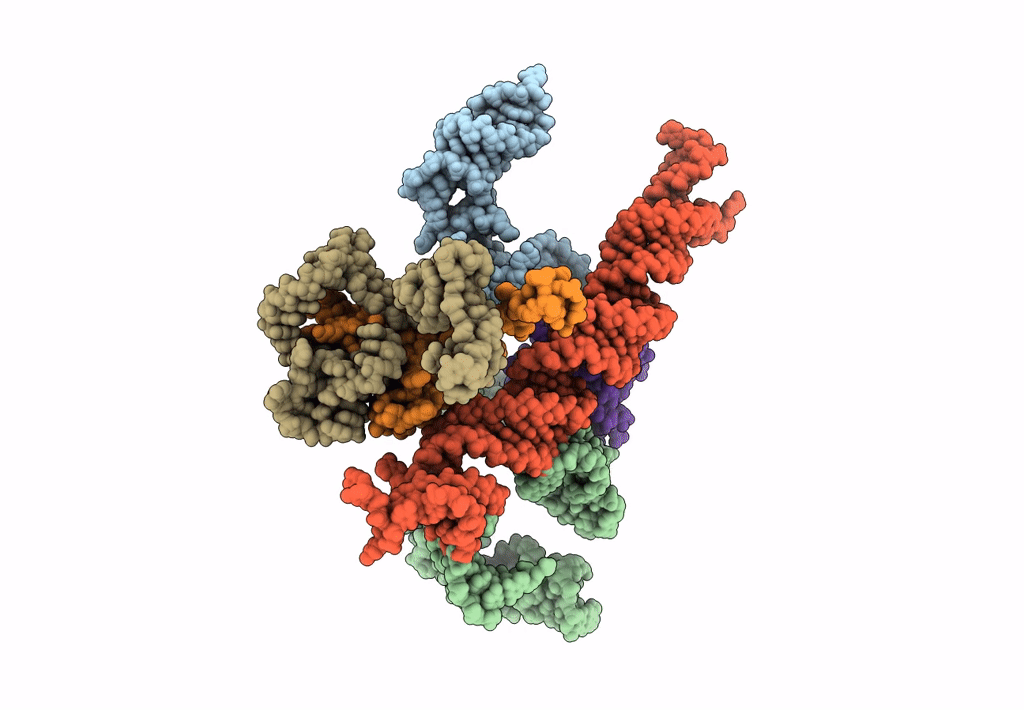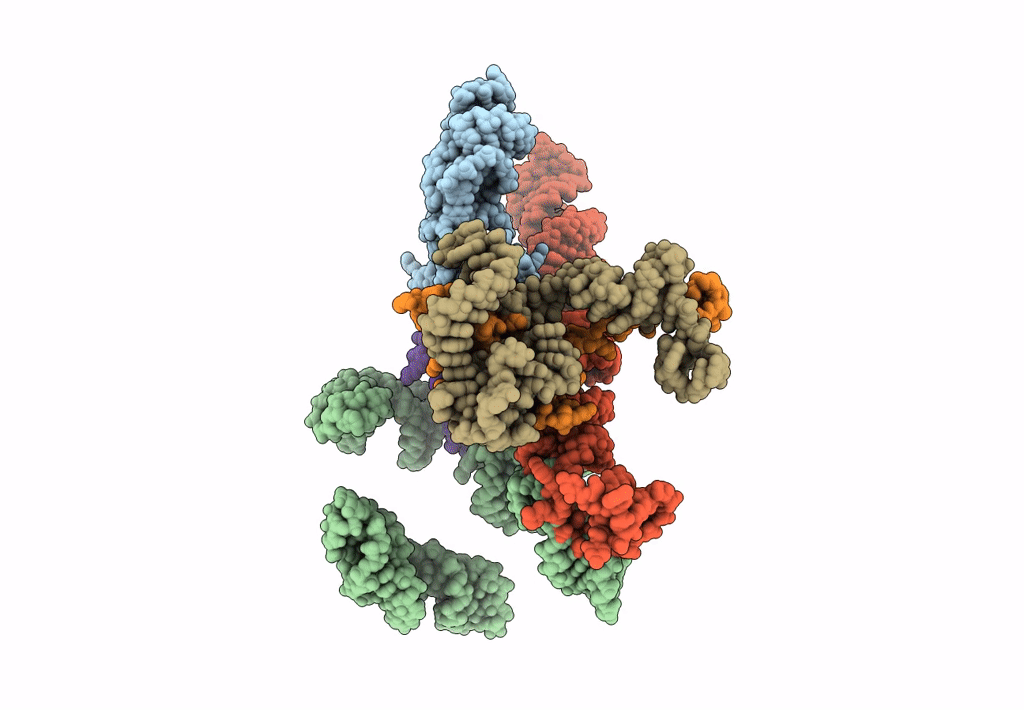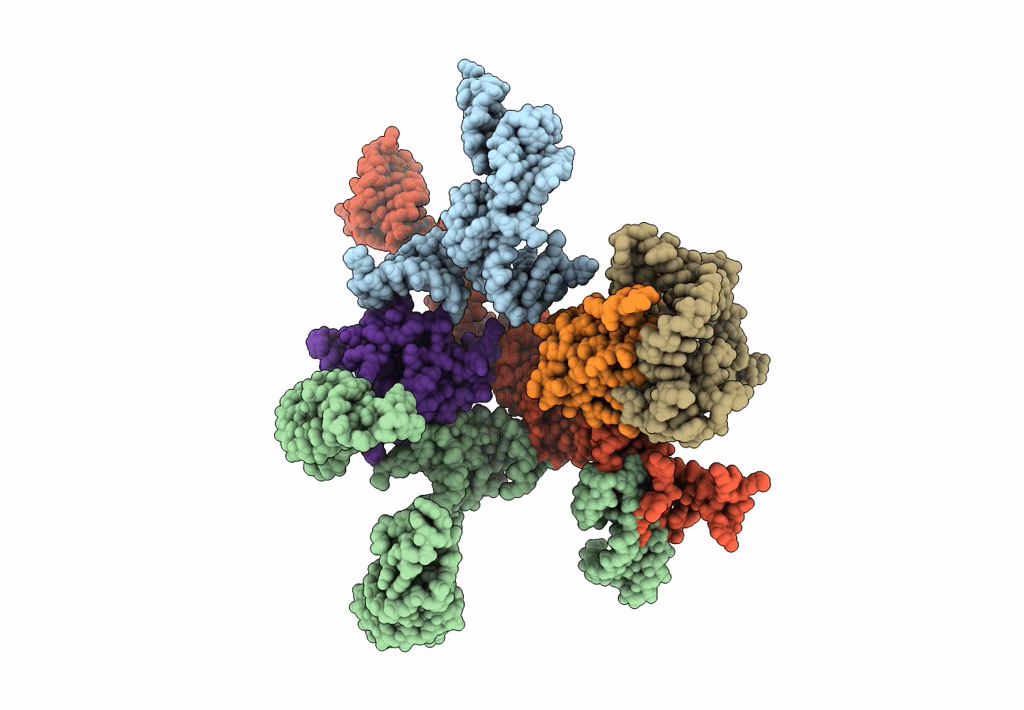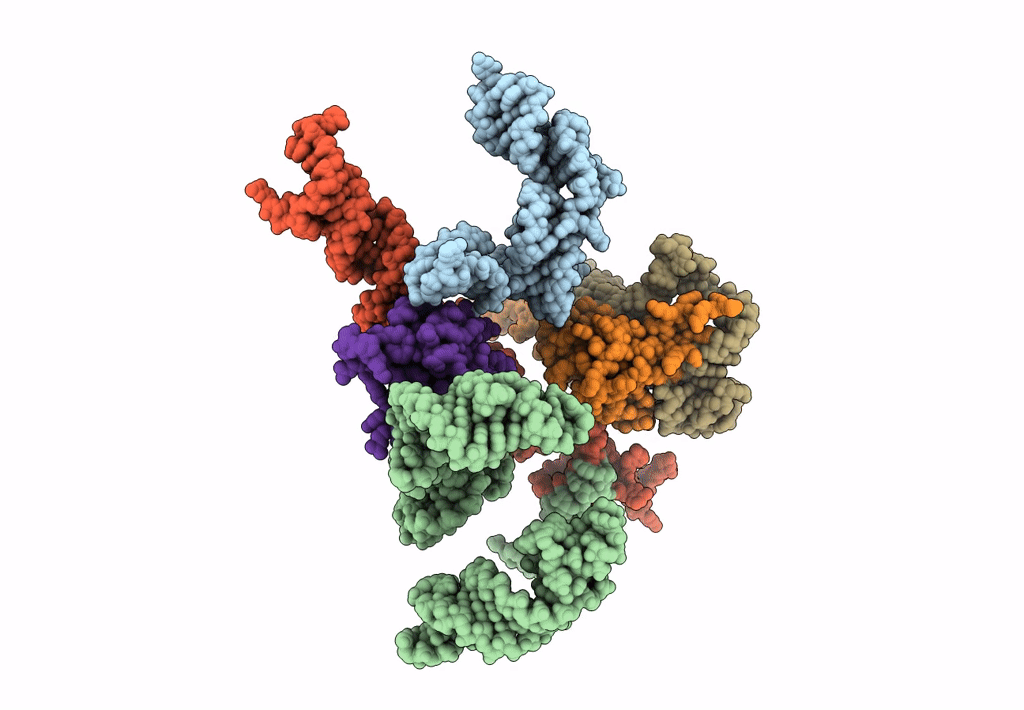
Title:
Models for ribosome components that are nearest neighbors to the bovine mitochondrial initiation factor2 bound to the E. Coli ribosome
Biological Source:
Source Organism(s):
Escherichia coli (Taxon ID: 562)
Method Details:
Experimental Method:
Resolution:
10.80 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE


