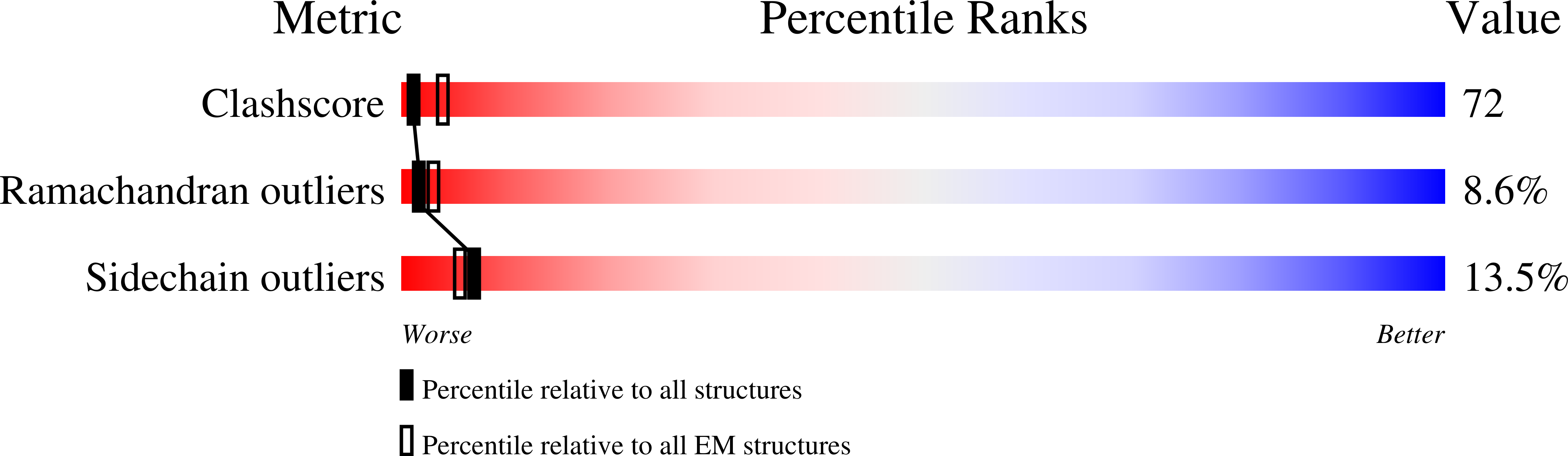
Deposition Date
2010-02-02
Release Date
2010-05-12
Last Version Date
2024-11-20
Entry Detail
PDB ID:
3IYL
Keywords:
Title:
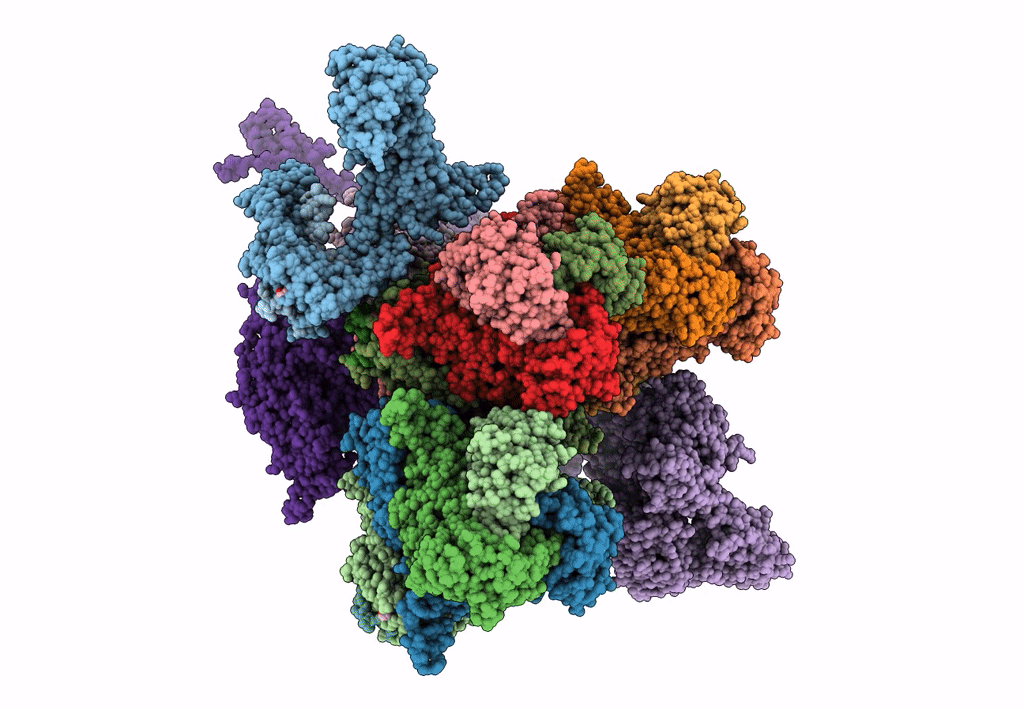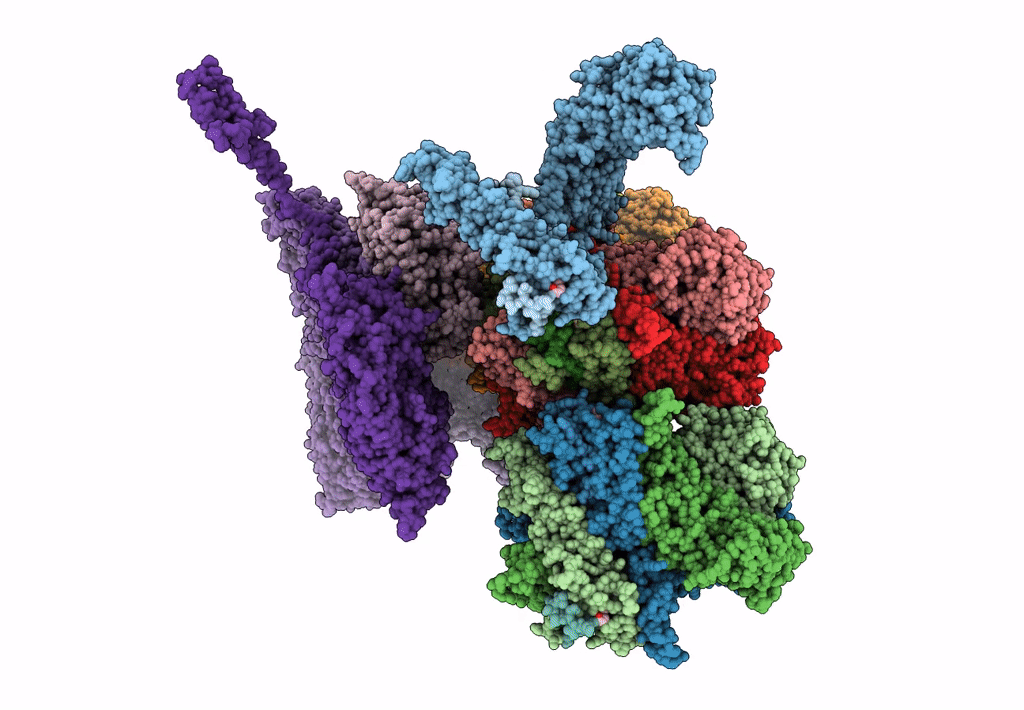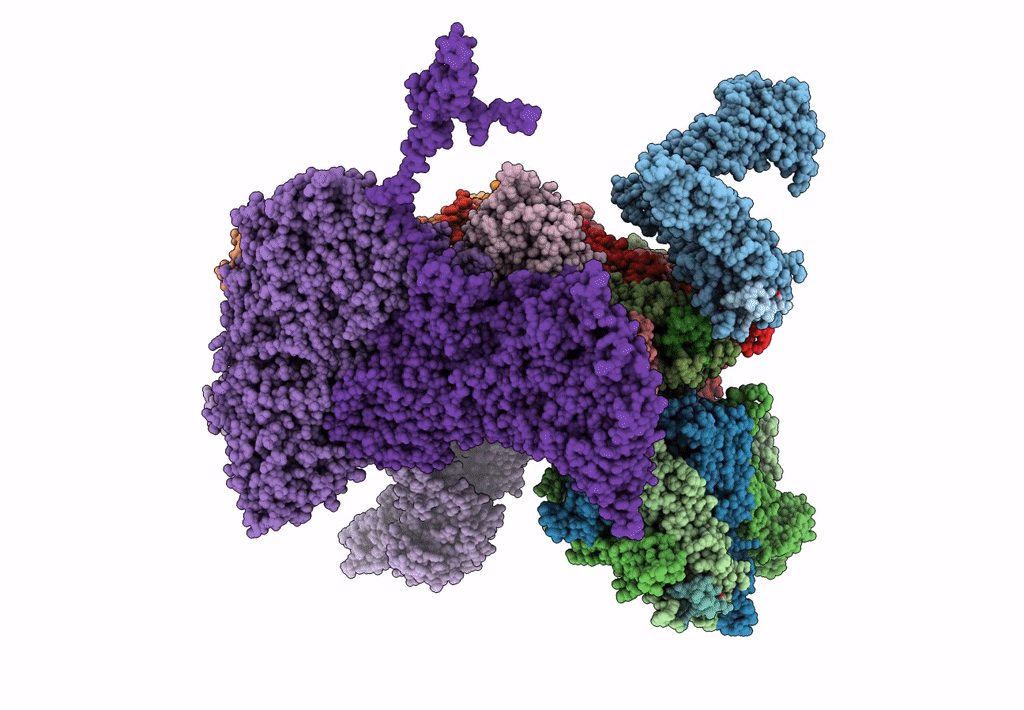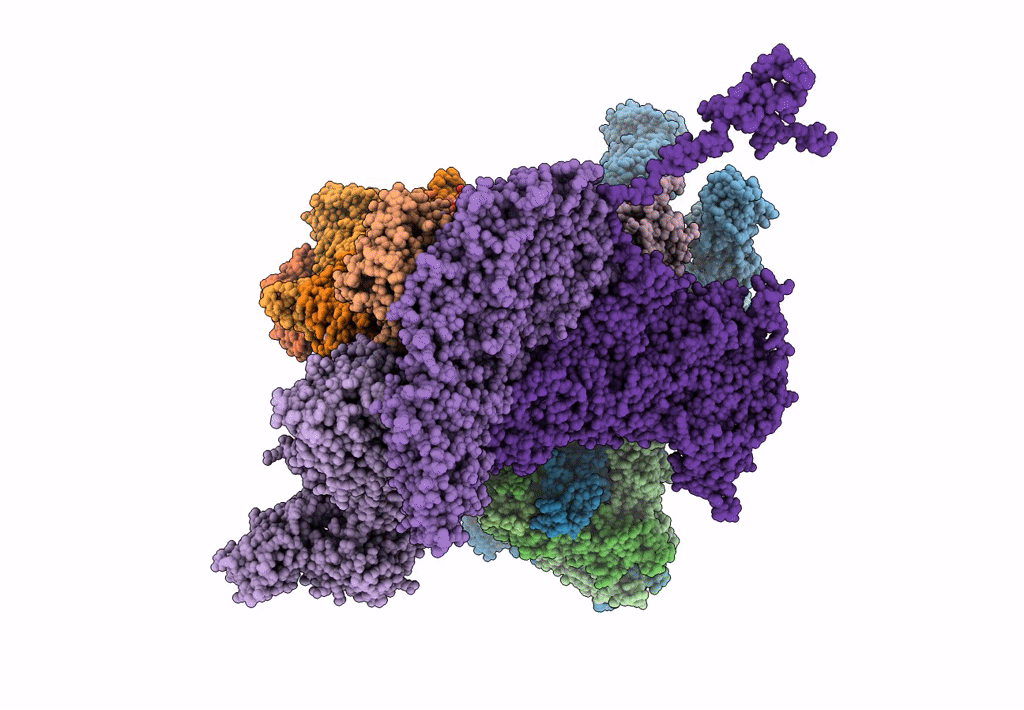
Atomic CryoEM Structure of a Nonenveloped Virus Suggests How Membrane Penetration Protein is Primed for Cell Entry
Biological Source:
Source Organism(s):
Grass carp reovirus (Taxon ID: 128987)
Method Details:
Experimental Method:
Resolution:
3.30 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE