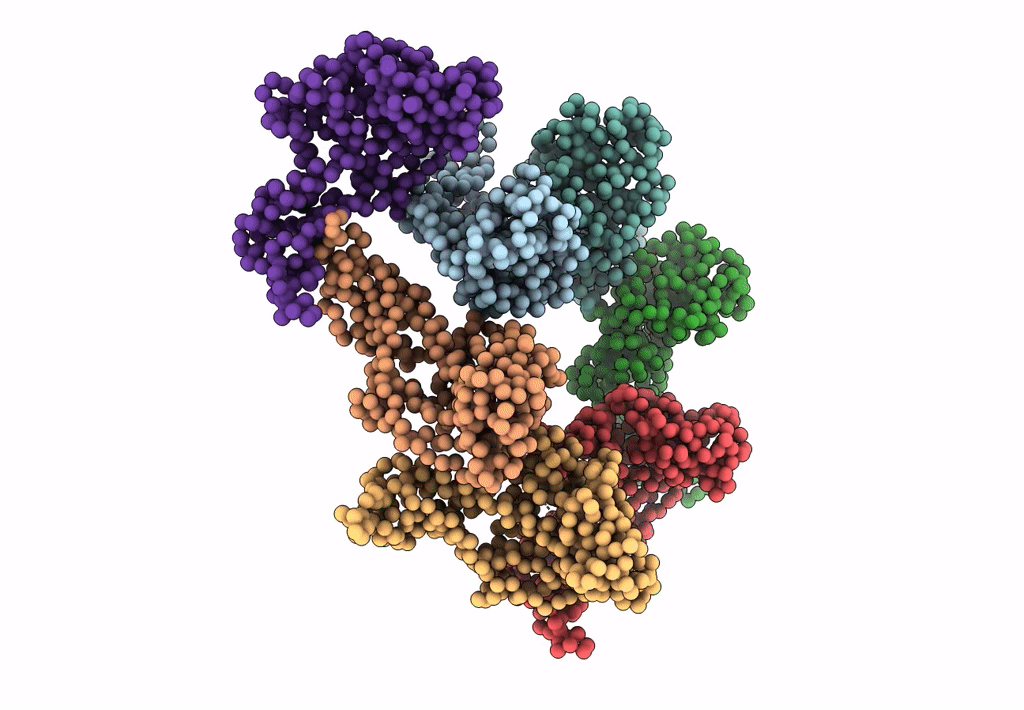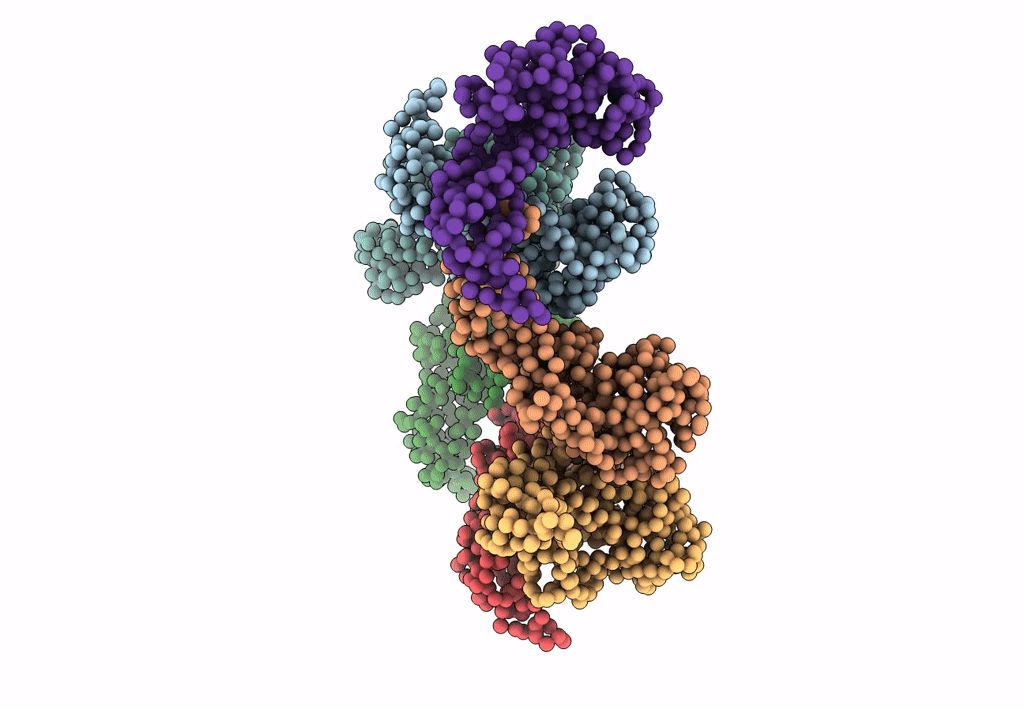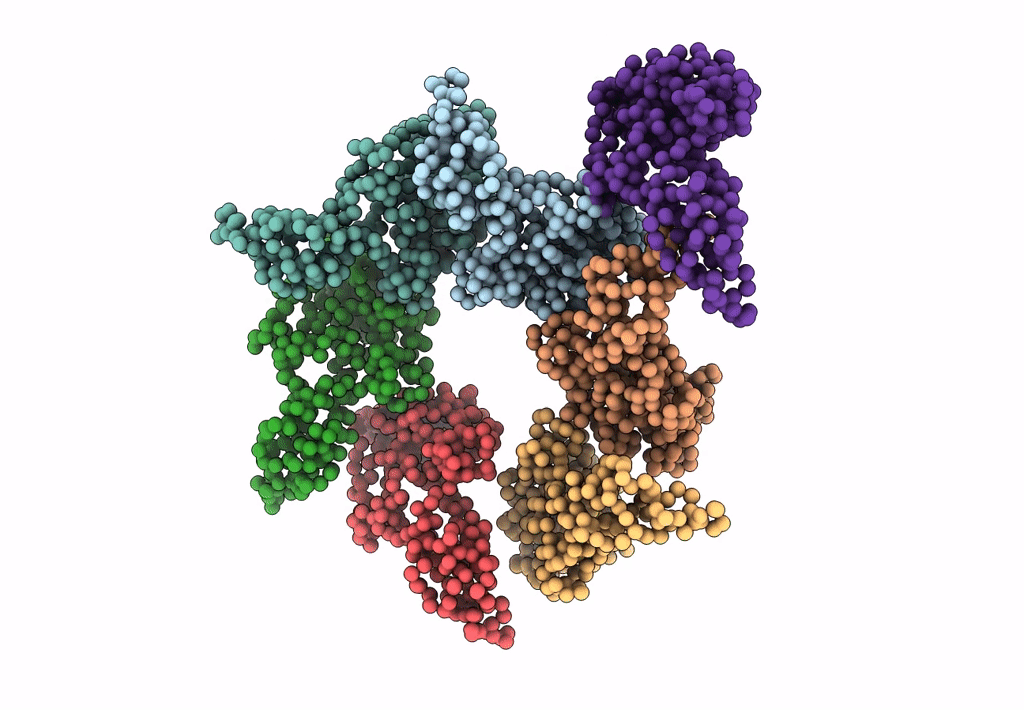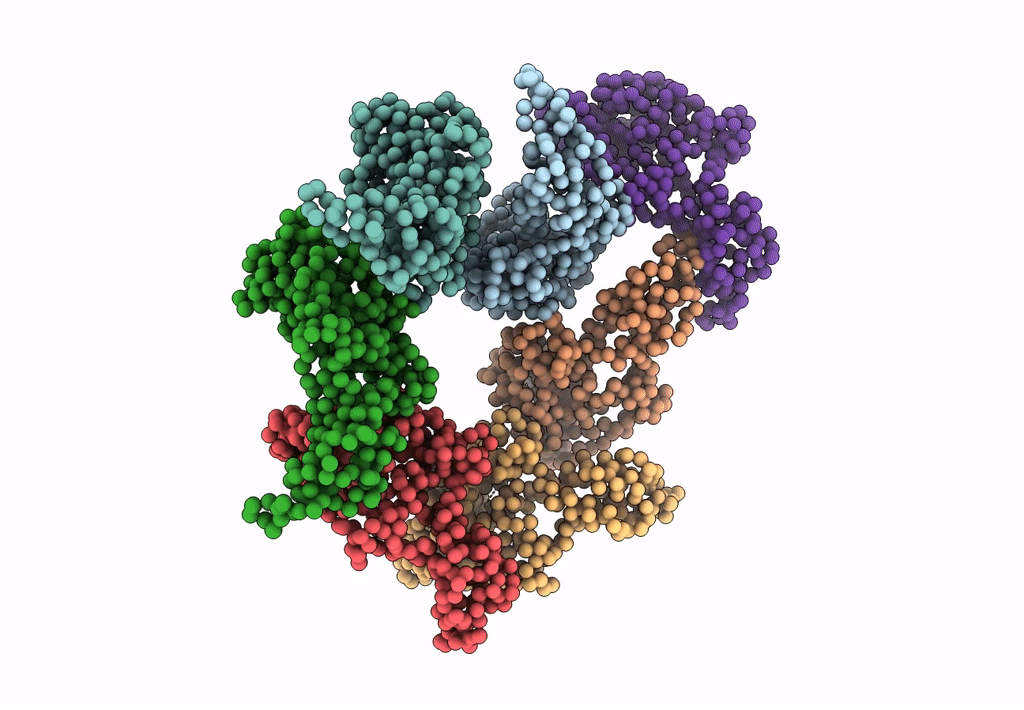
Deposition Date
2009-12-14
Release Date
2010-03-31
Last Version Date
2024-02-21
Entry Detail
PDB ID:
3IYI
Keywords:
Title:
P22 expanded head coat protein structures reveal a novel mechanism for capsid maturation: Stability without auxiliary proteins or chemical cross-links
Biological Source:
Source Organism(s):
Enterobacteria phage P22 (Taxon ID: 10754)
Method Details:
Experimental Method:
Resolution:
9.10 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE


