Deposition Date
2009-08-25
Release Date
2010-04-07
Last Version Date
2023-09-06
Entry Detail
PDB ID:
3IS9
Keywords:
Title:
Crystal structure of the HIV-1 reverse transcriptase (RT) in complex with the alkenyldiarylmethane (ADAM) Non-nucleoside RT Inhibitor dimethyl 3,3'-(6-methoxy-6-oxohex-1-ene-1,1-diyl)bis(5-cyano-6-methoxybenzoate).
Biological Source:
Source Organism(s):
Human immunodeficiency virus type 1 BH10 (Taxon ID: 11678)
Expression System(s):
Method Details:
Experimental Method:
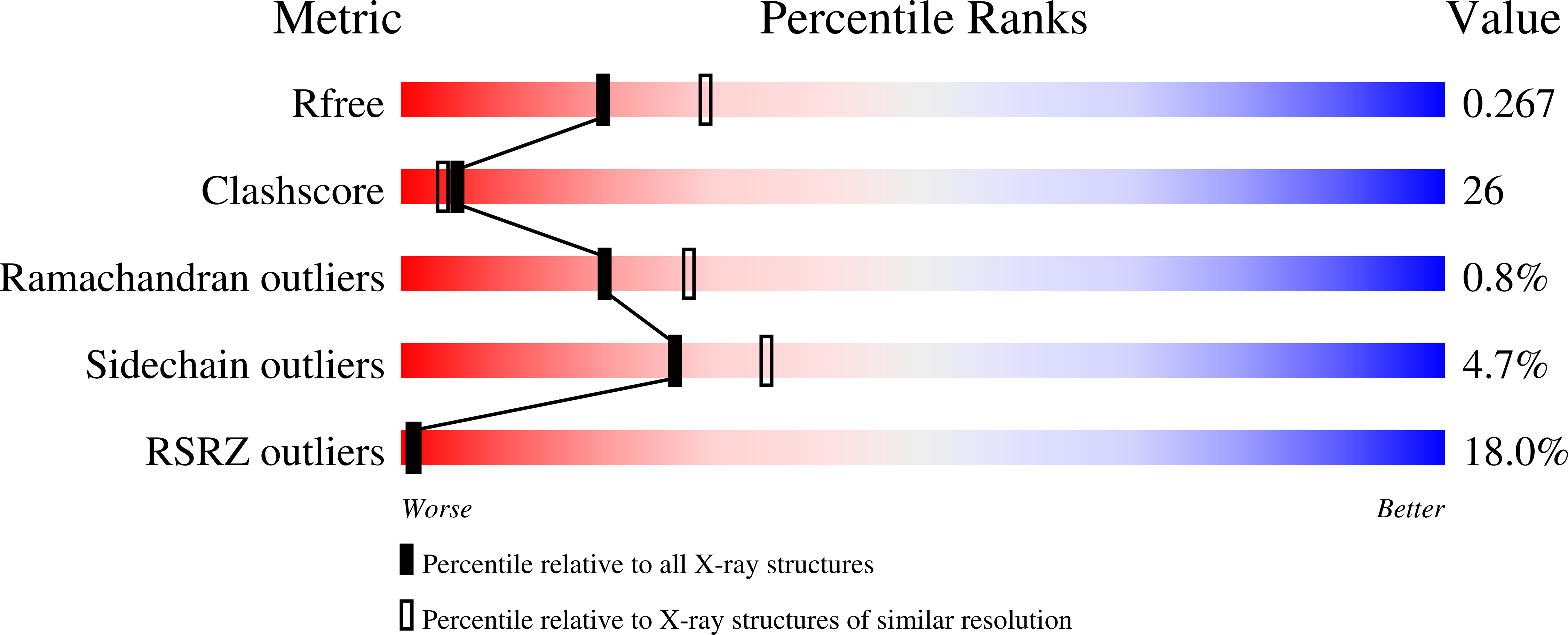
Resolution:
2.55 Å
R-Value Free:
0.28
R-Value Work:
0.25
R-Value Observed:
0.25
Space Group:
C 1 2 1