Deposition Date
2009-04-17
Release Date
2009-05-05
Last Version Date
2023-02-01
Entry Detail
PDB ID:
3H41
Keywords:
Title:
CRYSTAL STRUCTURE OF A NLPC/P60 FAMILY PROTEIN (BCE_2878) FROM BACILLUS CEREUS ATCC 10987 AT 1.79 A RESOLUTION
Biological Source:
Source Organism(s):
Bacillus cereus ATCC 10987 (Taxon ID: 222523)
Expression System(s):
Method Details:
Experimental Method:
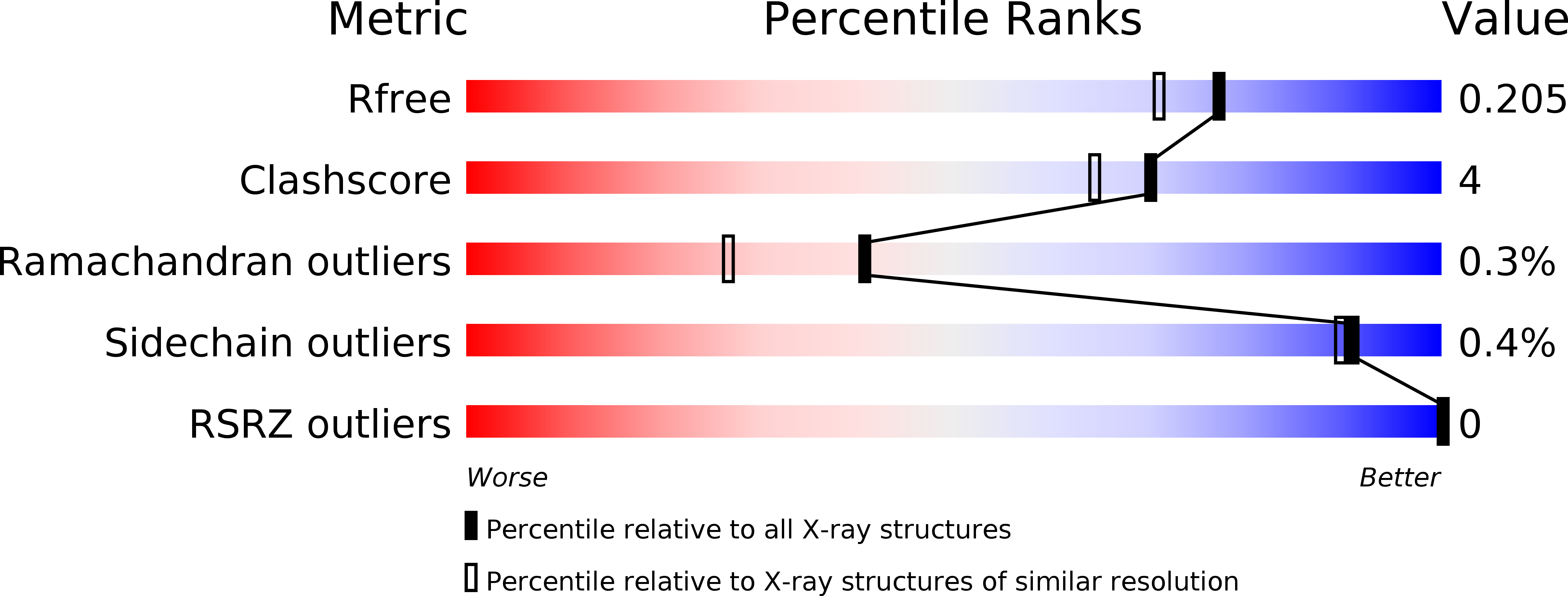
Resolution:
1.79 Å
R-Value Free:
0.19
R-Value Work:
0.16
R-Value Observed:
0.16
Space Group:
C 1 2 1