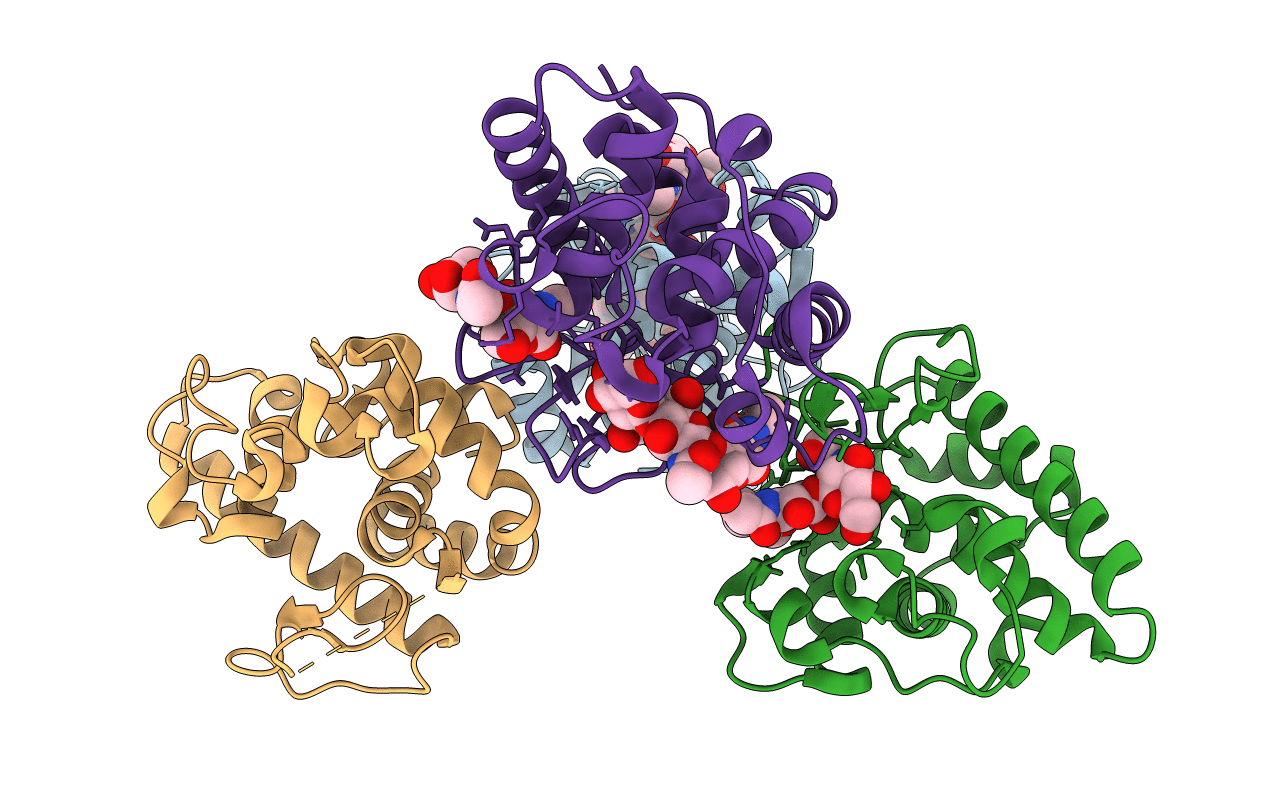
Deposition Date
2009-04-02
Release Date
2009-10-20
Last Version Date
2023-11-01
Entry Detail
PDB ID:
3GXR
Keywords:
Title:
The crystal structure of g-type lysozyme from Atlantic cod (Gadus morhua L.) in complex with NAG oligomers sheds new light on substrate binding and the catalytic mechanism. Structure with NAG to 1.7
Biological Source:
Source Organism(s):
Gadus morhua (Taxon ID: 8049)
Expression System(s):
Method Details:
Experimental Method:
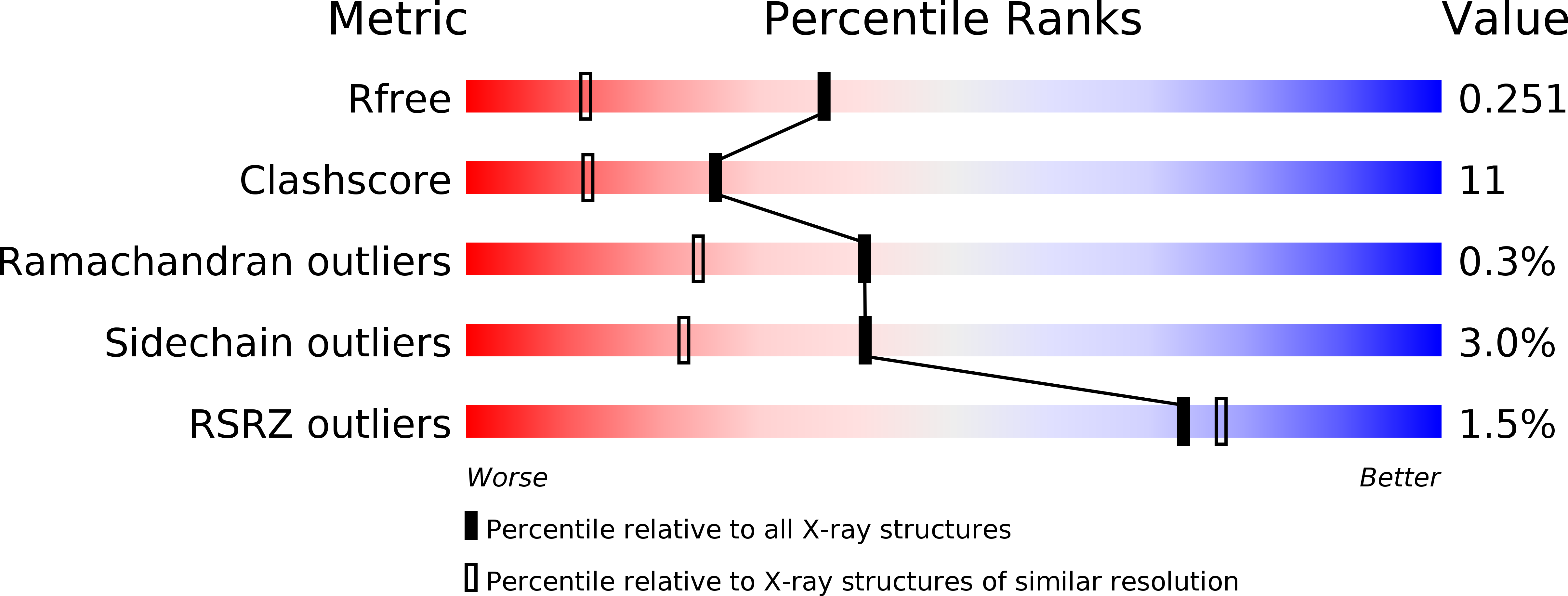
Resolution:
1.70 Å
R-Value Free:
0.25
R-Value Work:
0.20
R-Value Observed:
0.20
Space Group:
C 1 2 1