Deposition Date
2009-03-16
Release Date
2009-07-28
Last Version Date
2023-09-06
Entry Detail
PDB ID:
3GN9
Keywords:
Title:
Crystal structure of the major pseudopilin from the type 2 secretion system of Vibrio vulnificus
Biological Source:
Source Organism:
Vibrio vulnificus (Taxon ID: 672)
Host Organism:
Method Details:
Experimental Method:
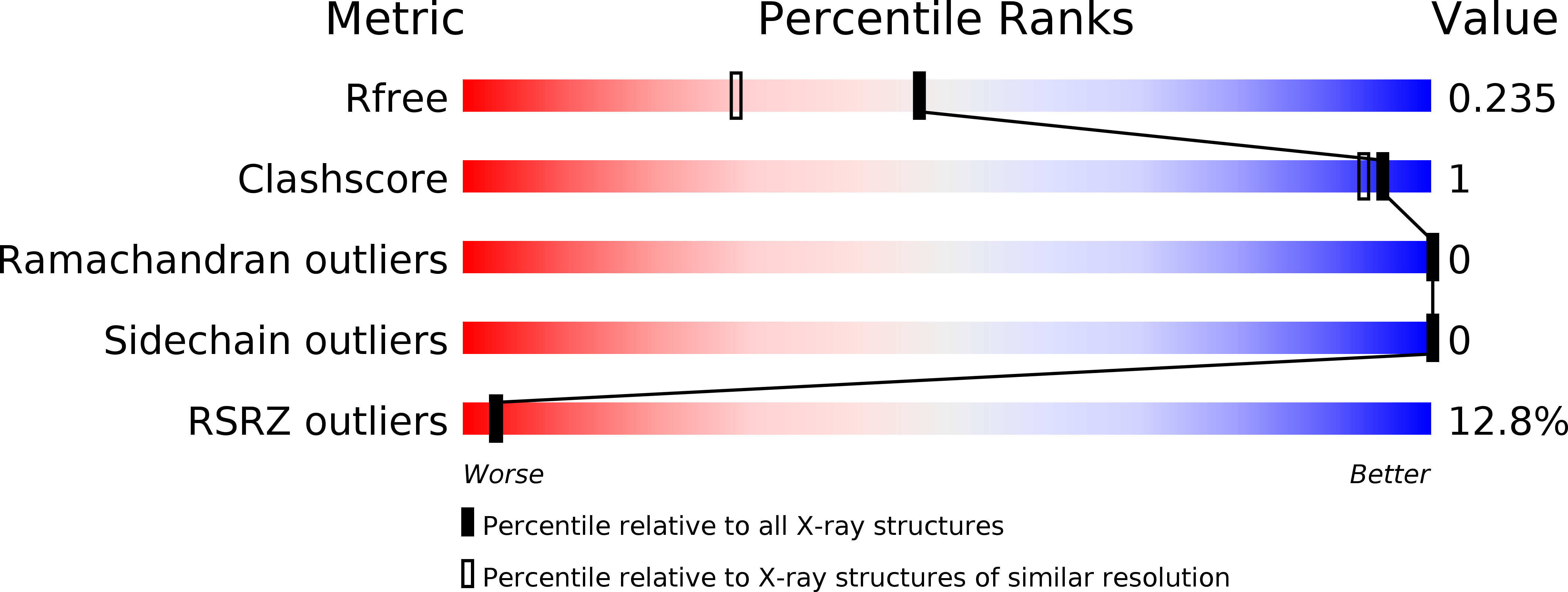
Resolution:
1.86 Å
R-Value Free:
0.20
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
C 1 2 1