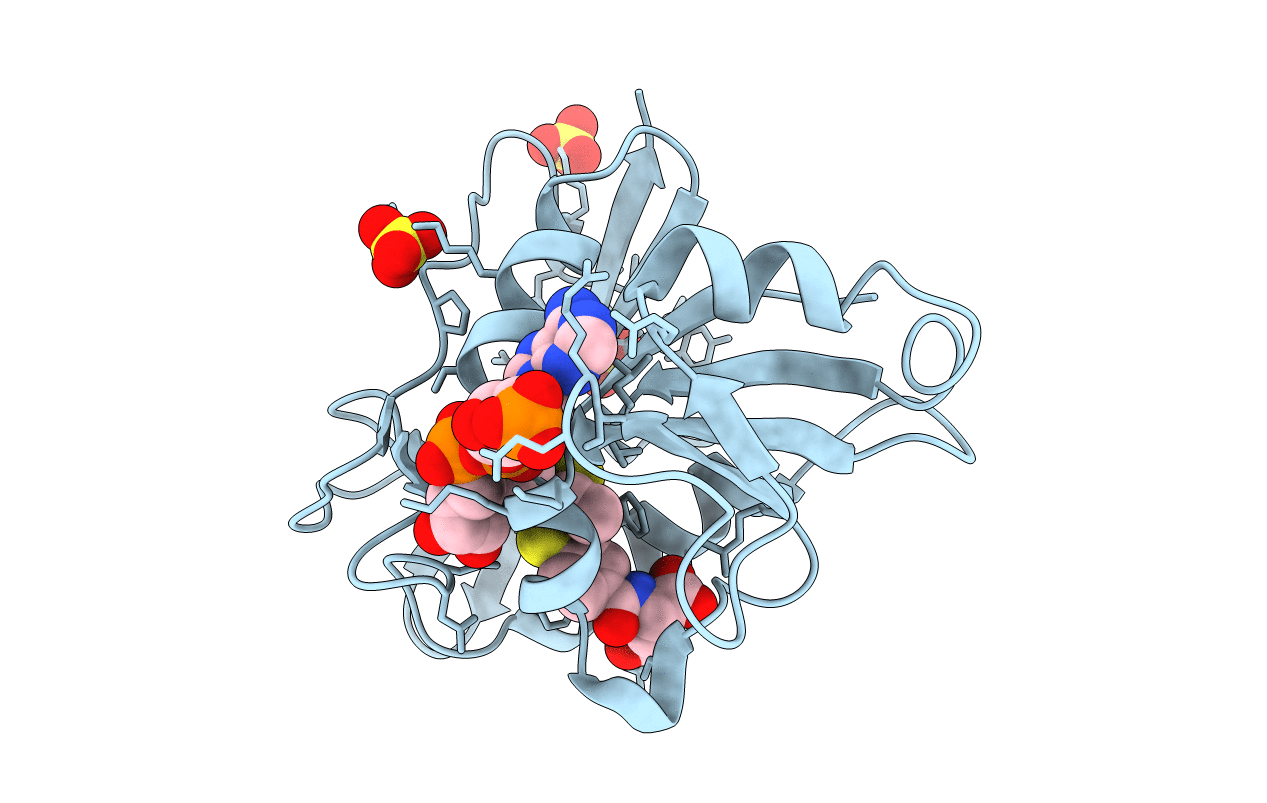
Deposition Date
2009-03-03
Release Date
2009-09-22
Last Version Date
2023-09-06
Entry Detail
PDB ID:
3GHC
Keywords:
Title:
Design, Synthesis, and X-ray Crystal Structure of Classical and Nonclassical 2-amino-4-oxo-5-substituted-6-thieno[2,3-d]pyrimidines as dual thymidylate synthase and dihydrofolate reductase inhibitors and as potential antitumor agenst
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
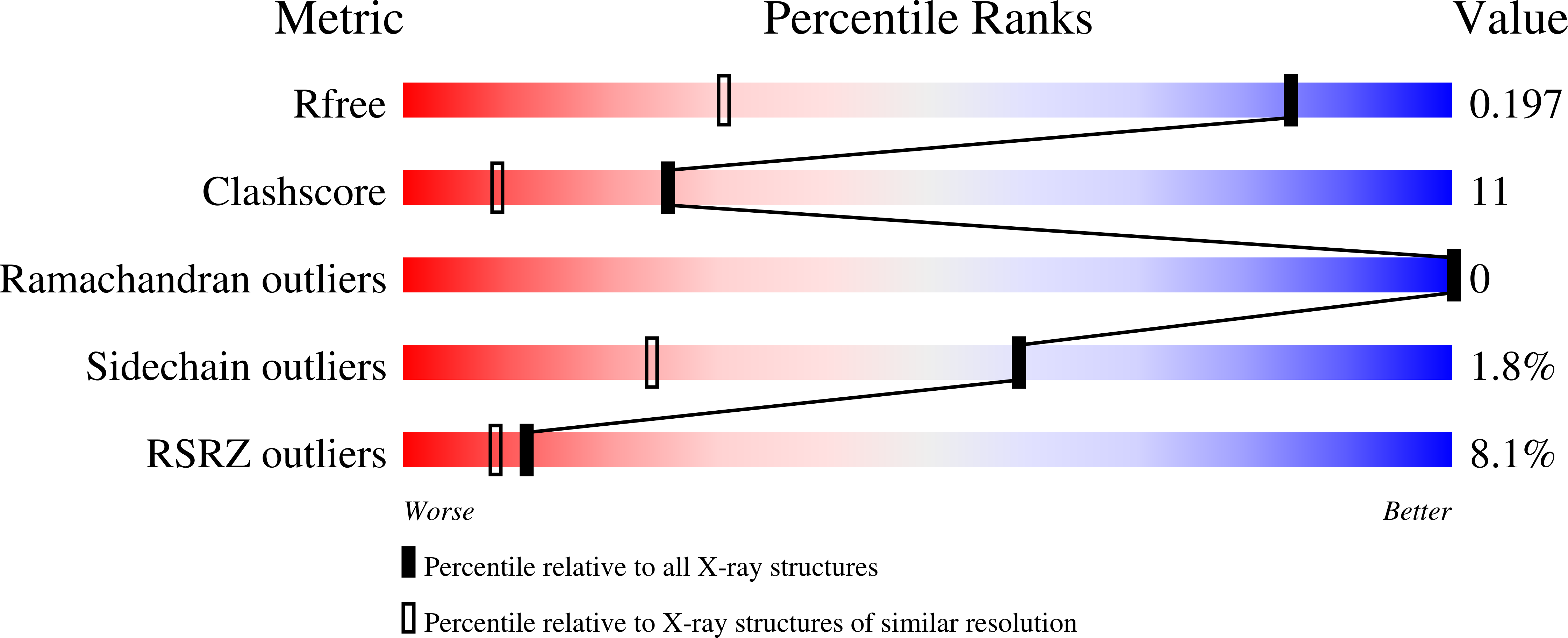
Resolution:
1.30 Å
R-Value Free:
0.19
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
H 3