Deposition Date
1990-09-04
Release Date
1991-10-15
Last Version Date
2024-10-16
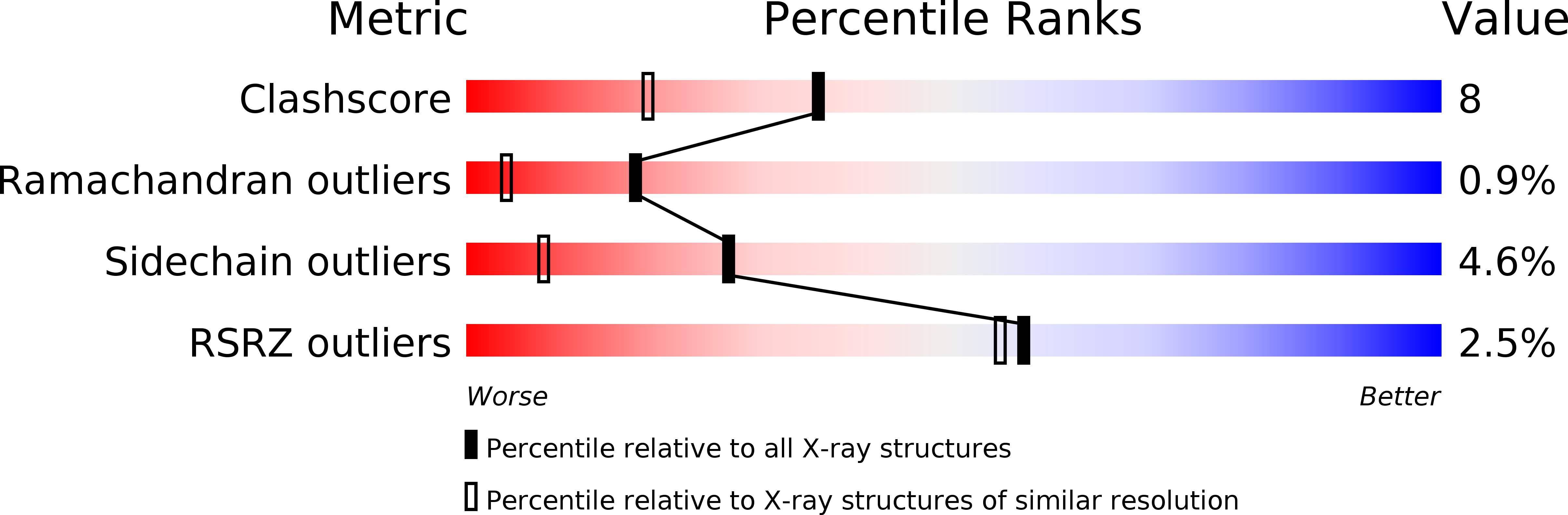
Entry Detail
PDB ID:
3GCT
Keywords:
Title:
STRUCTURE OF GAMMA-*CHYMOTRYPSIN IN THE RANGE $P*H 2.0 TO $P*H 10.5 SUGGESTS THAT GAMMA-CHYMOTRYPSIN IS A COVALENT ACYL-ENZYME ADDUCT AT LOW $P*H
Biological Source:
Source Organism(s):
Bos taurus (Taxon ID: 9913)
Method Details: