Deposition Date
2009-01-07
Release Date
2009-03-03
Last Version Date
2024-11-20
Entry Detail
PDB ID:
3FQW
Keywords:
Title:
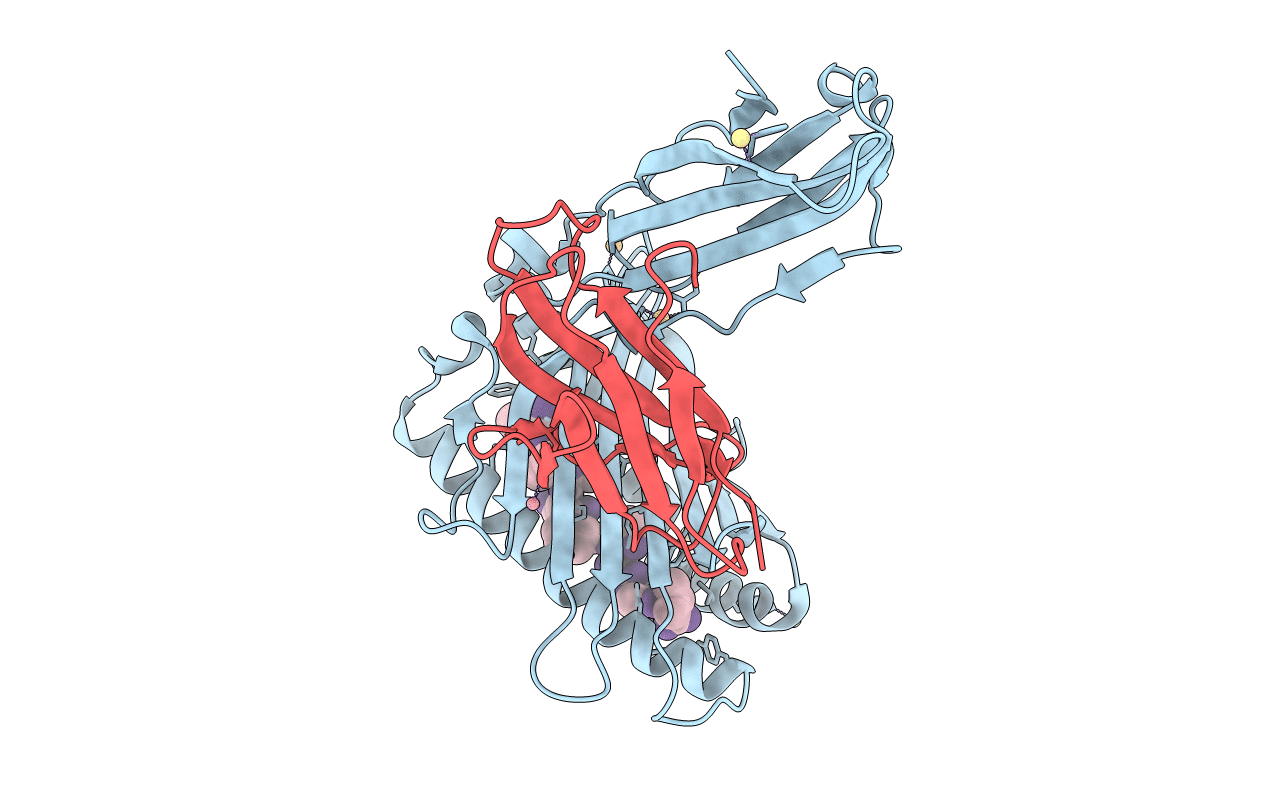
Phosphorylation of self-peptides alters Human Leukocyte Antigen Class I-restricted antigen presentation and generates tumor specific epitopes
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
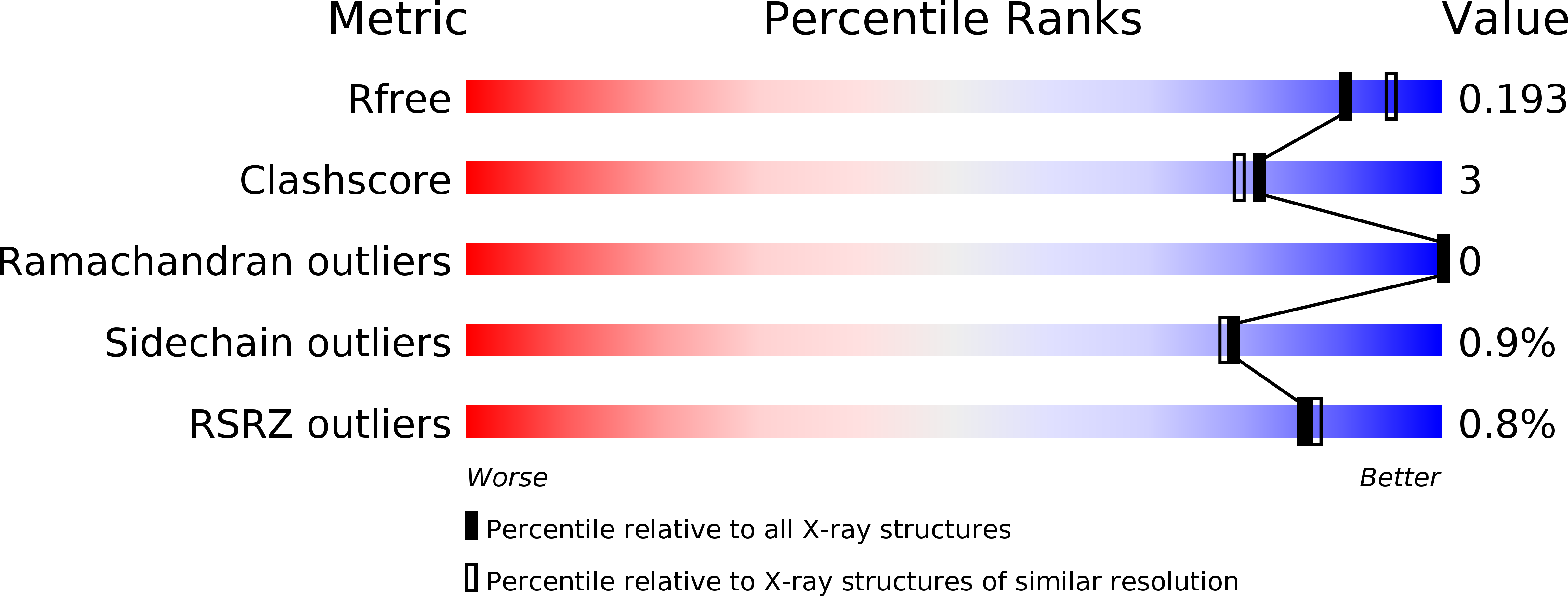
Resolution:
1.93 Å
R-Value Free:
0.19
R-Value Work:
0.16
R-Value Observed:
0.17
Space Group:
P 21 21 21