Deposition Date
2009-01-05
Release Date
2009-04-07
Last Version Date
2025-03-19
Entry Detail
PDB ID:
3FPB
Keywords:
Title:
The Structure of Sarcoplasmic Reticulum Ca2+-ATPase Bound To Cyclopiazonic acid with ATP
Biological Source:
Source Organism(s):
Oryctolagus cuniculus (Taxon ID: 9986)
Method Details:
Experimental Method:
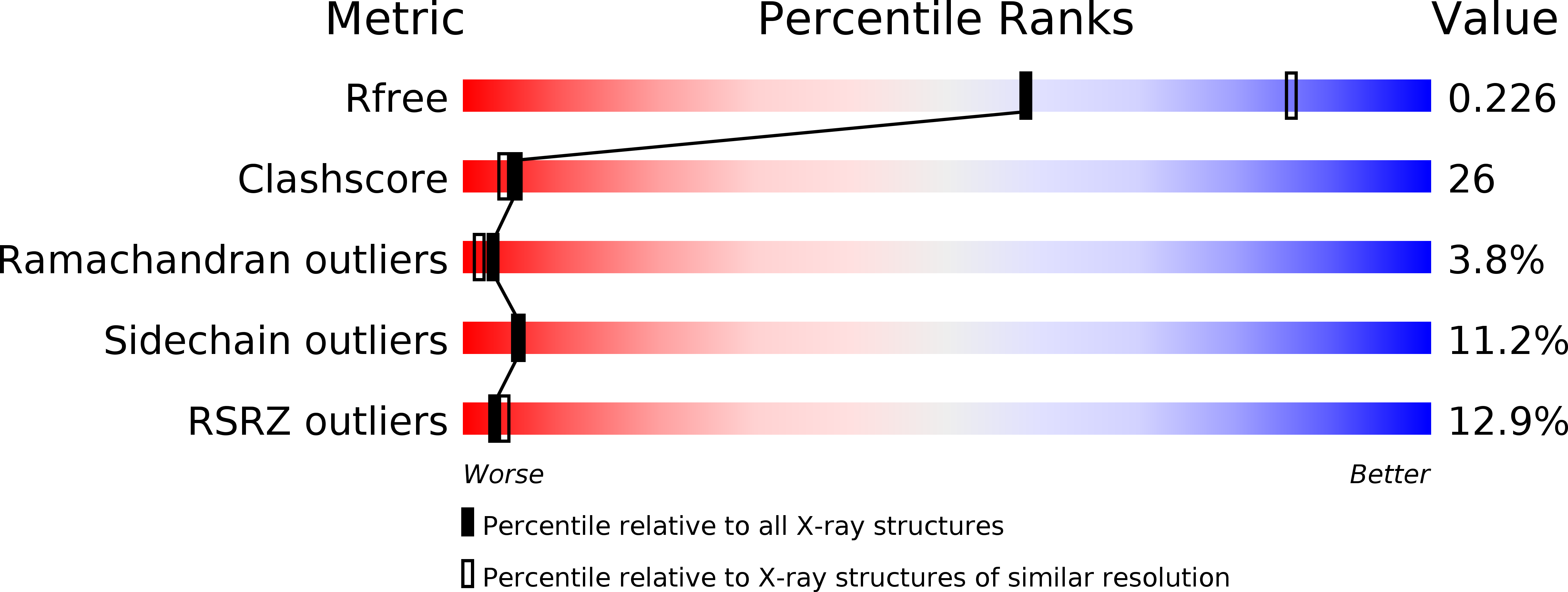
Resolution:
2.55 Å
R-Value Free:
0.23
R-Value Work:
0.18
R-Value Observed:
0.18
Space Group:
C 1 2 1