Deposition Date
2008-11-14
Release Date
2008-12-30
Last Version Date
2023-12-27
Entry Detail
PDB ID:
3F9V
Keywords:
Title:
Crystal Structure Of A Near Full-Length Archaeal MCM: Functional Insights For An AAA+ Hexameric Helicase
Biological Source:
Source Organism(s):
Sulfolobus solfataricus (Taxon ID: 2287)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
4.35 Å
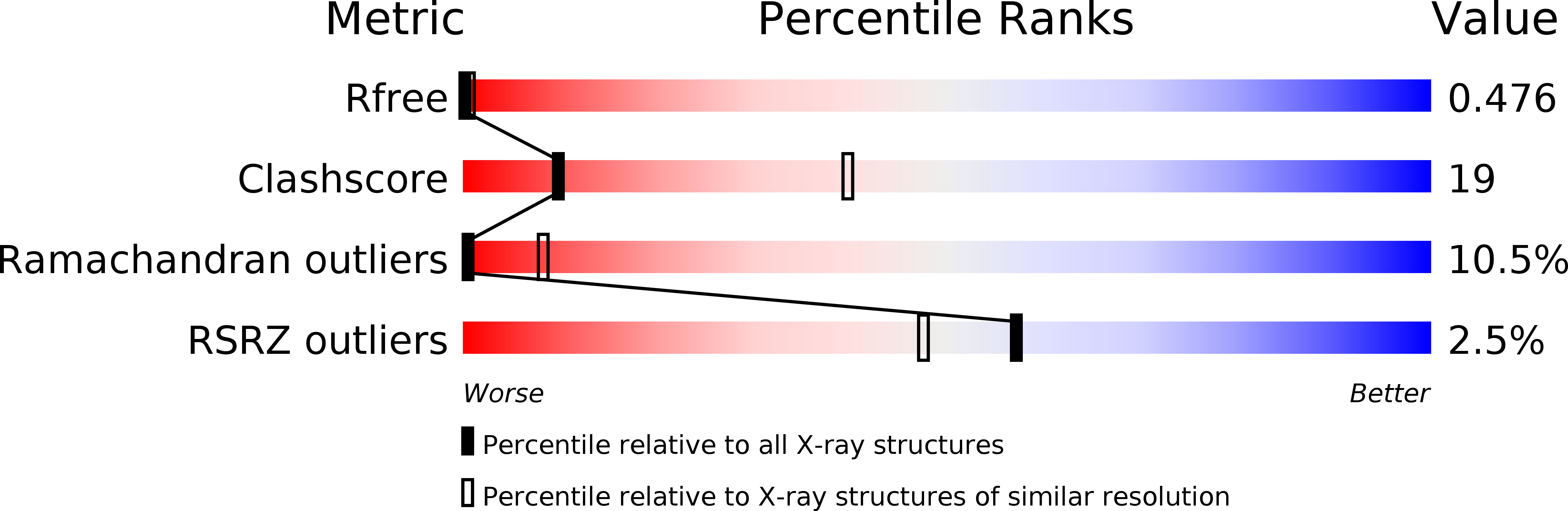
R-Value Free:
0.48
R-Value Work:
0.41
R-Value Observed:
0.41
Space Group:
I 41 2 2