Deposition Date
2008-11-11
Release Date
2009-07-21
Last Version Date
2023-11-15
Entry Detail
PDB ID:
3F87
Keywords:
Title:
An alpha/beta-Peptide Helix Bundle with a Pure beta-Amino Acid Core and a Distinctive Quarternary Structure: GCN4pLI derivative with beta residues at a and d heptad positions - higher symmetry crystal
Method Details:
Experimental Method:
Resolution:
2.40 Å
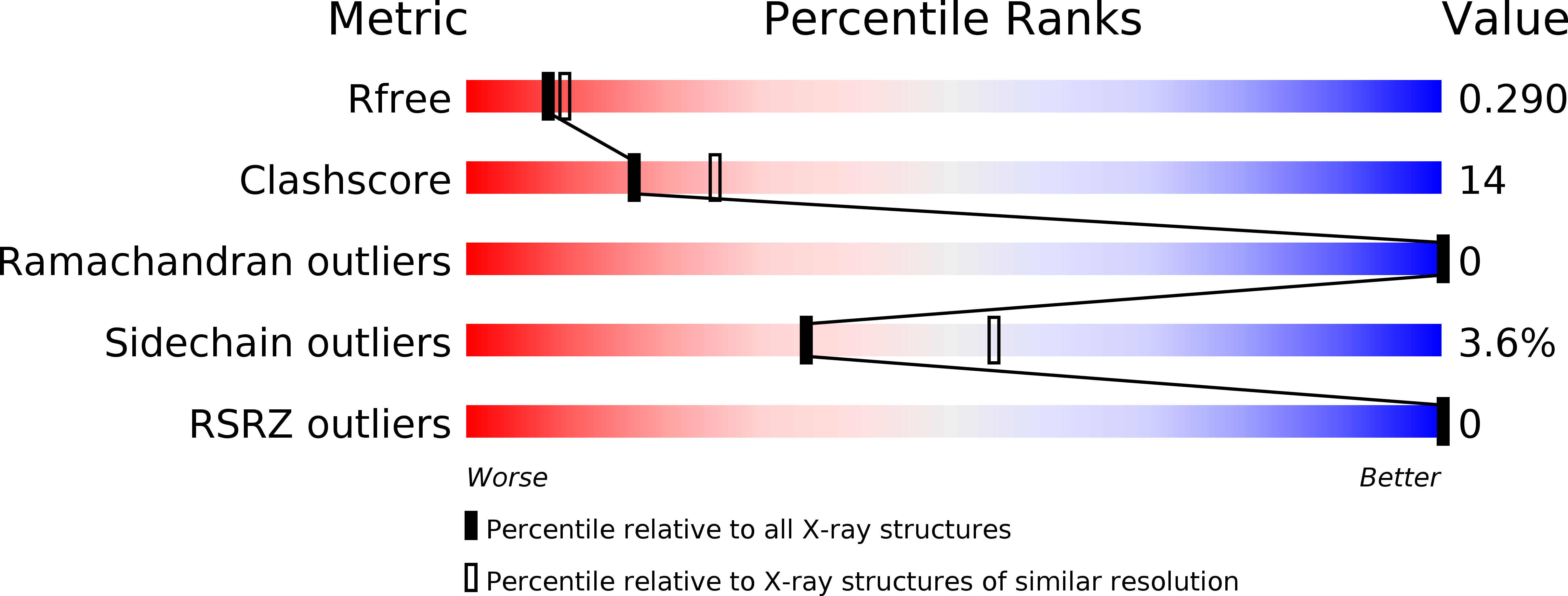
R-Value Free:
0.29
R-Value Work:
0.23
R-Value Observed:
0.23
Space Group:
P 43 21 2