Deposition Date
2008-11-03
Release Date
2009-09-15
Last Version Date
2024-10-30
Entry Detail
PDB ID:
3F4Y
Keywords:
Title:
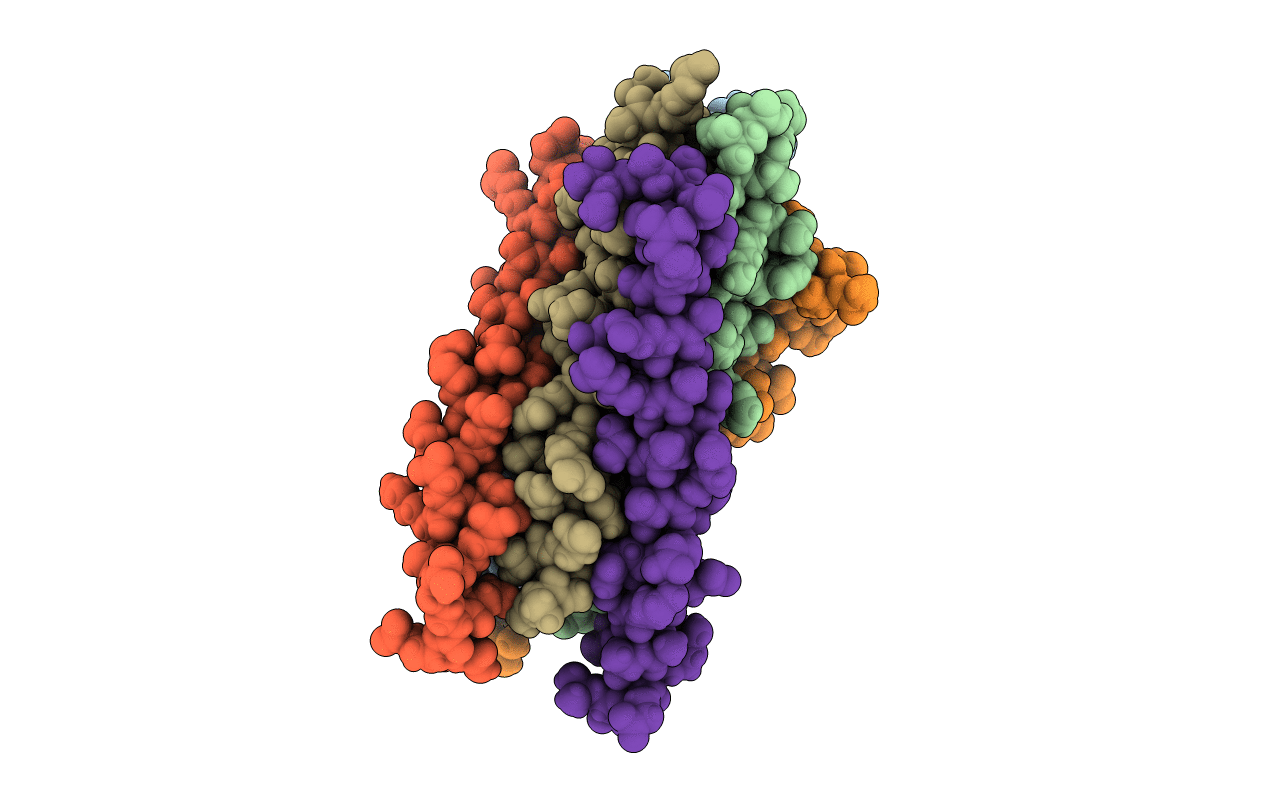
HIV gp41 six-helix bundle containing a mutant CHR alpha-peptide sequence
Method Details:
Experimental Method:
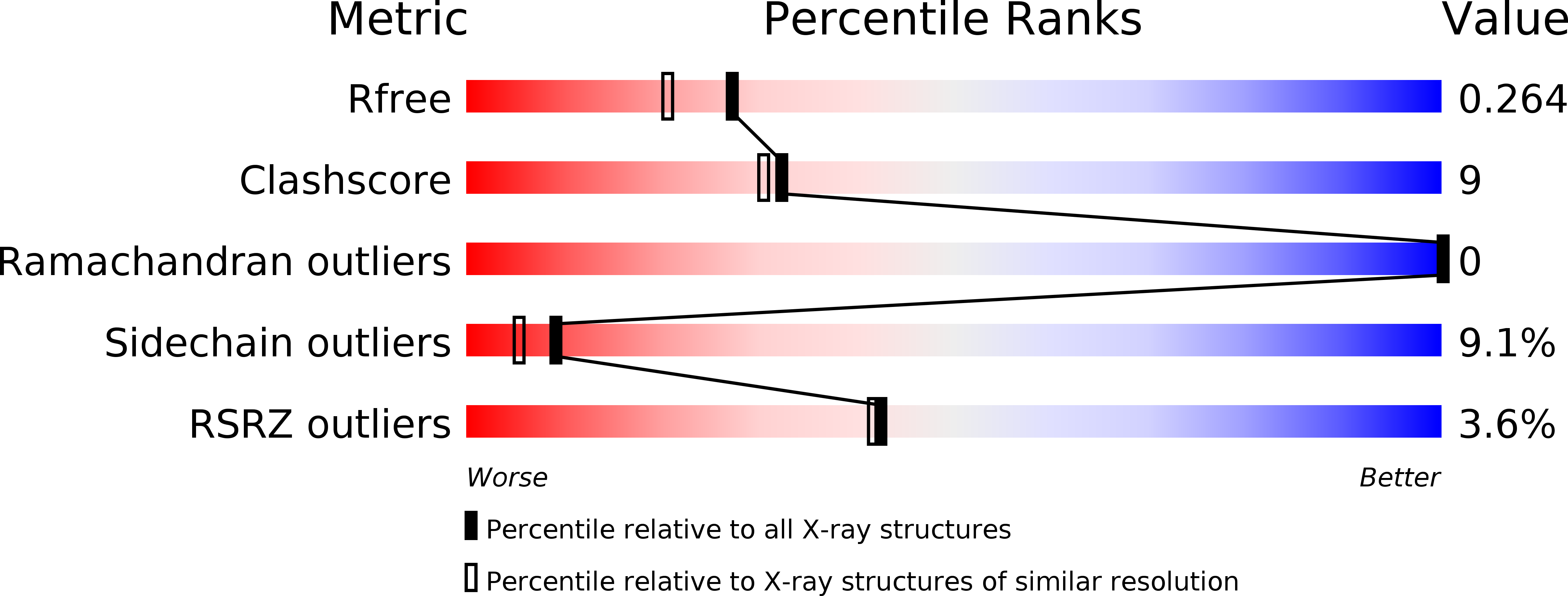
Resolution:
2.00 Å
R-Value Free:
0.26
R-Value Work:
0.20
R-Value Observed:
0.21
Space Group:
P 21 21 2