Deposition Date
2008-10-25
Release Date
2009-10-06
Last Version Date
2024-04-03
Entry Detail
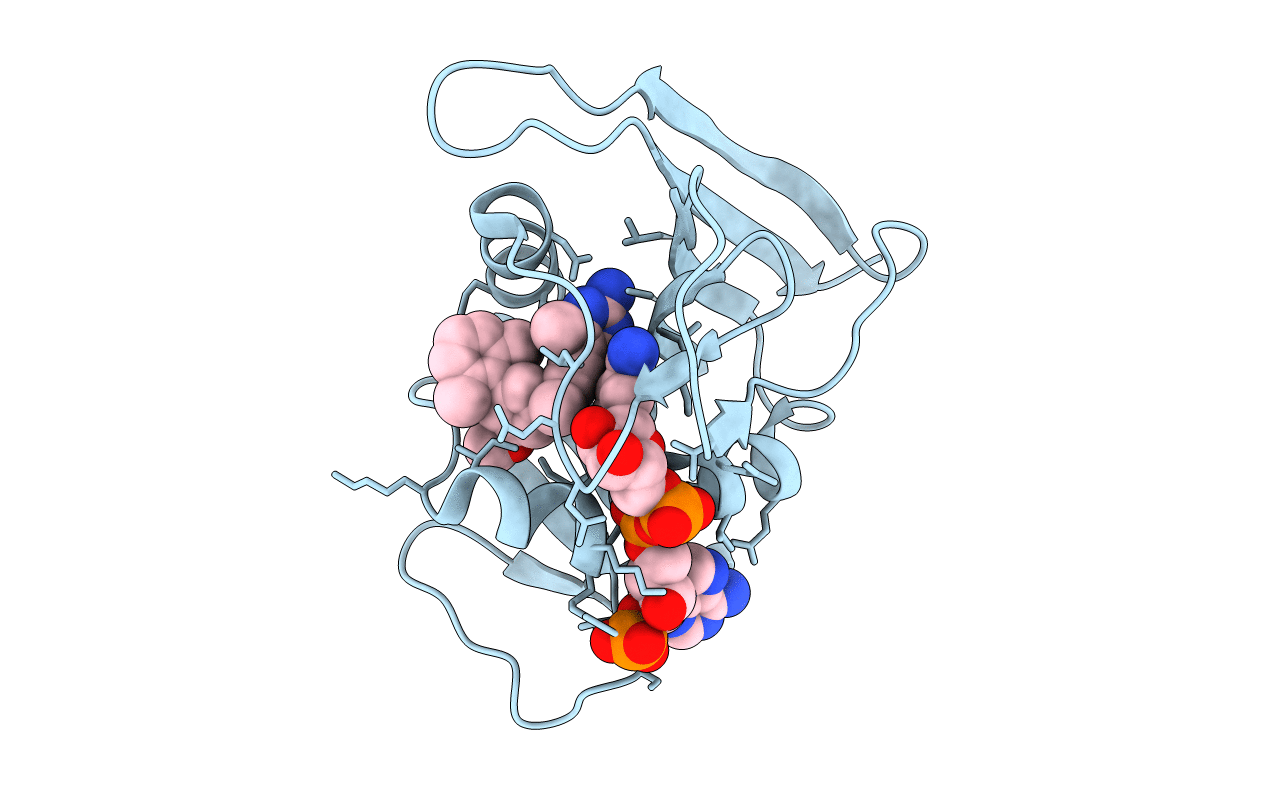
PDB ID:
3F0Q
Keywords:
Title:
Staphylococcus aureus dihydrofolate reductase complexed with NADPH and 2,4-Diamino-5-[3-(3-methoxy-5-(2,6-dimethylphenyl)phenyl)but-1-ynyl]-6-methylpyrimidine
Biological Source:
Source Organism(s):
Staphylococcus aureus RF122 (Taxon ID: 273036)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.08 Å
R-Value Free:
0.23
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 61 2 2


