Deposition Date
2008-09-29
Release Date
2008-11-04
Last Version Date
2024-02-21
Entry Detail
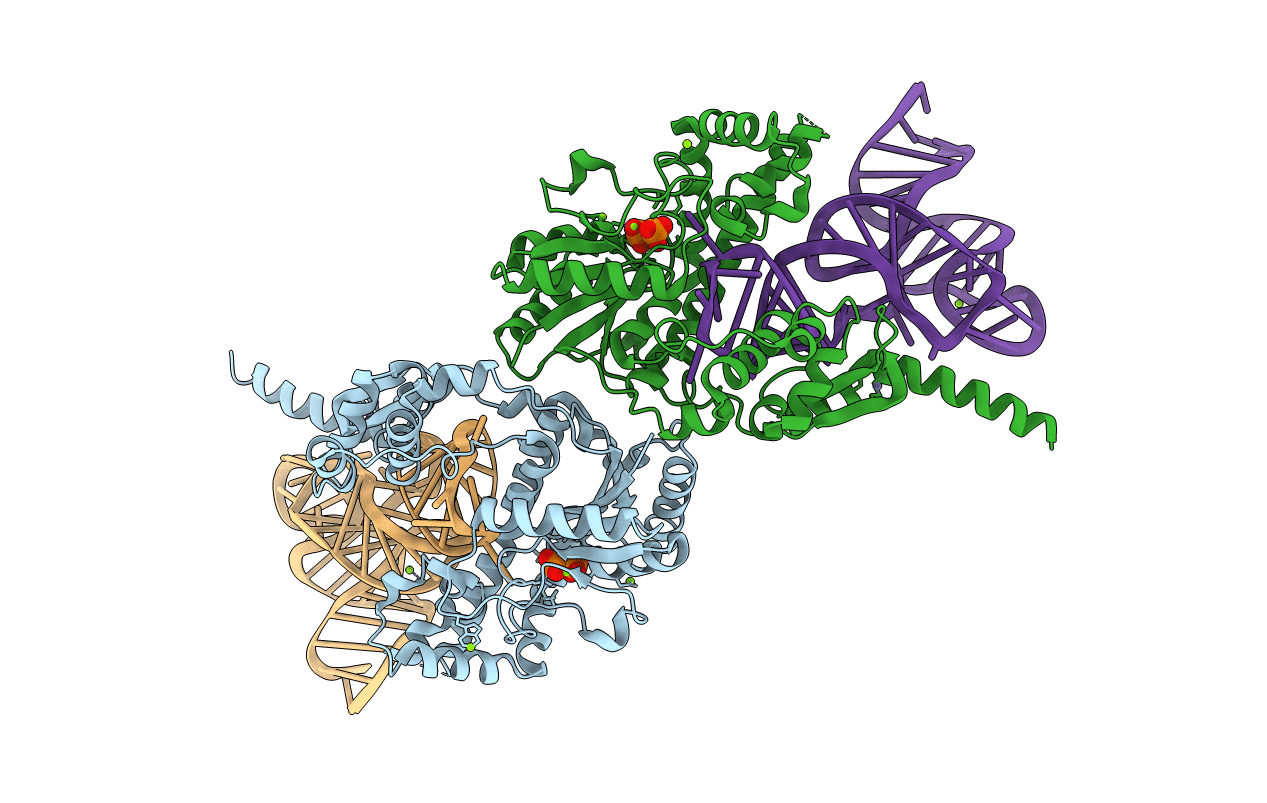
PDB ID:
3EPH
Keywords:
Title:
Crystallographic snapshots of eukaryotic dimethylallyltransferase acting on tRNA: Insight into tRNA recognition and reaction mechanism
Biological Source:
Source Organism(s):
Saccharomyces cerevisiae (Taxon ID: 4932)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.95 Å
R-Value Free:
0.23
R-Value Work:
0.19
Space Group:
C 2 2 21


