Deposition Date
2008-07-16
Release Date
2008-11-11
Last Version Date
2024-03-20
Entry Detail
PDB ID:
3DU1
Keywords:
Title:
The 2.0 Angstrom Resolution Crystal Structure of HetL, a Pentapeptide Repeat Protein involved in Heterocyst Differentiation Regulation from the Cyanobacterium Nostoc sp. Strain PCC 7120
Biological Source:
Source Organism(s):
Nostoc sp. (Taxon ID: 103690)
Expression System(s):
Method Details:
Experimental Method:
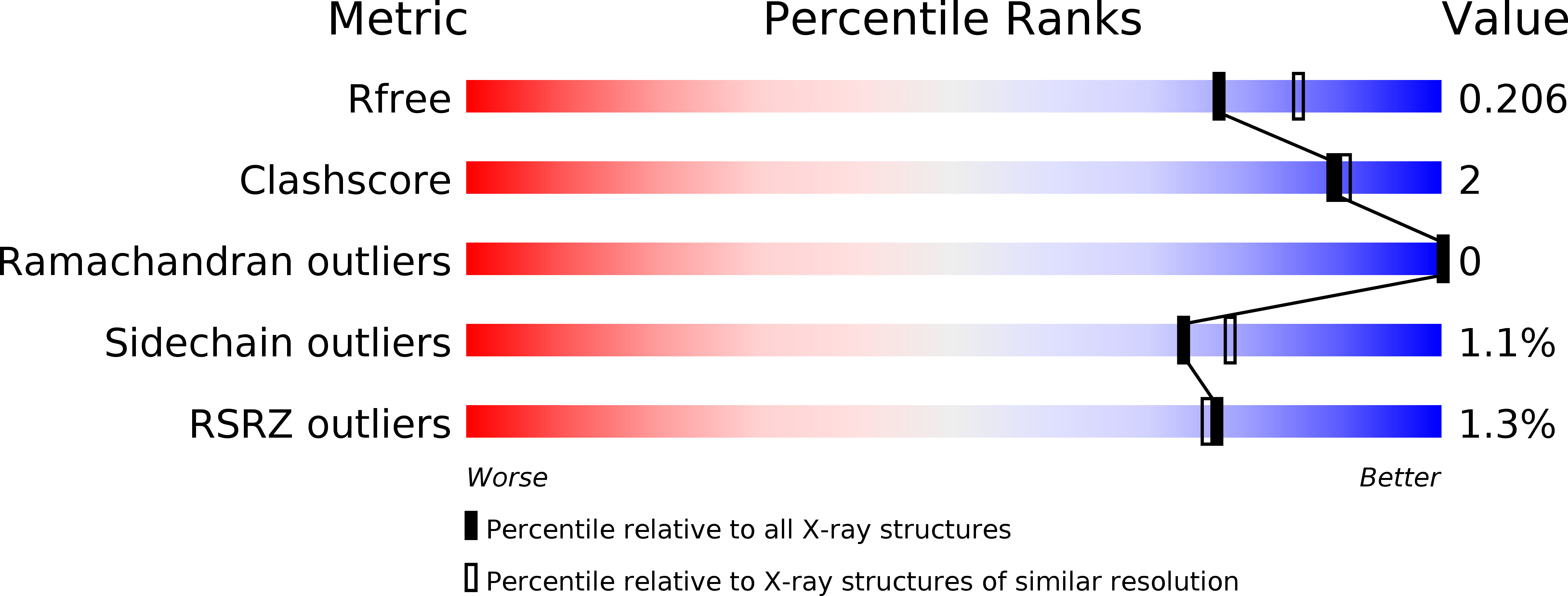
Resolution:
2.00 Å
R-Value Free:
0.20
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
I 2 2 2