Deposition Date
2008-07-12
Release Date
2009-05-19
Last Version Date
2024-10-30
Entry Detail
PDB ID:
3DSG
Keywords:
Title:
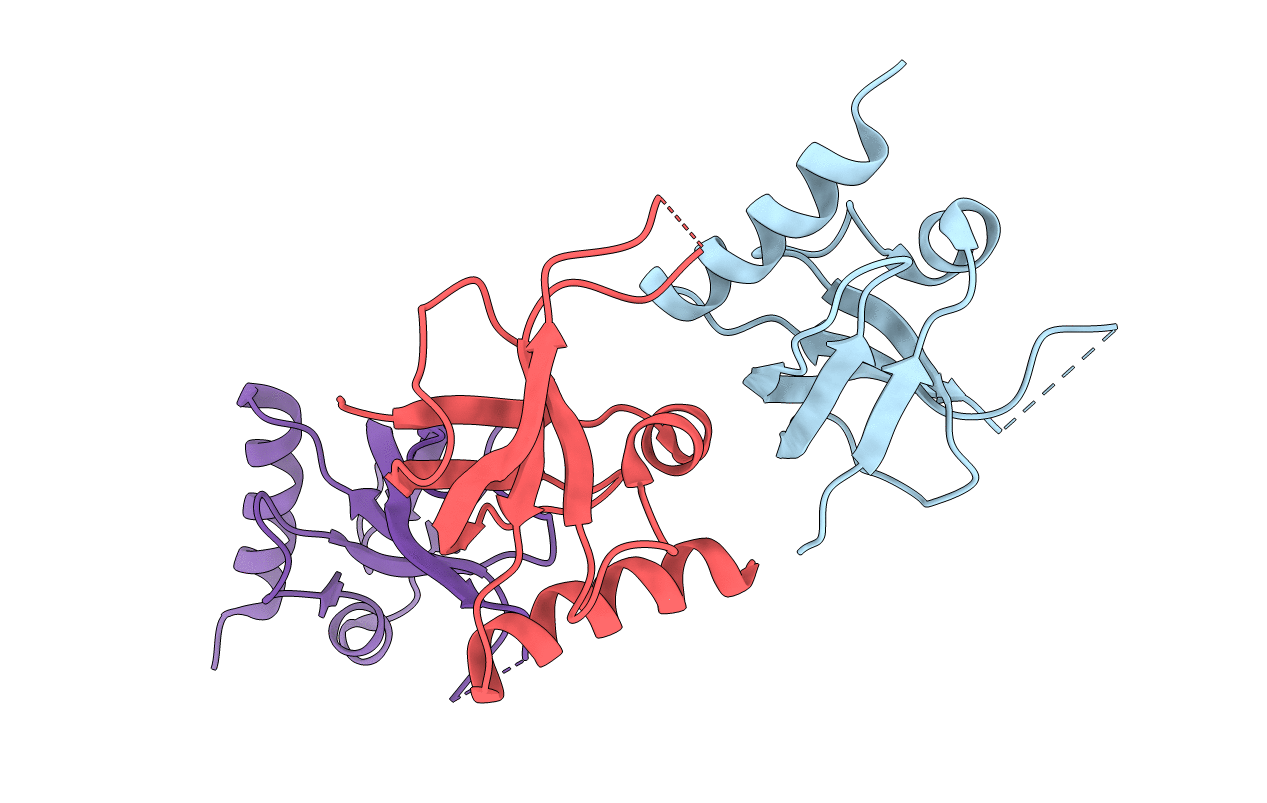
XC1028 from Xanthomonas campestris Adopts a PilZ Domain-like Structure Yet with Trivial c-di-GMP Binding Activity
Biological Source:
Source Organism(s):
Xanthomonas campestris pv. campestris (Taxon ID: 340)
Expression System(s):
Method Details:
Experimental Method:
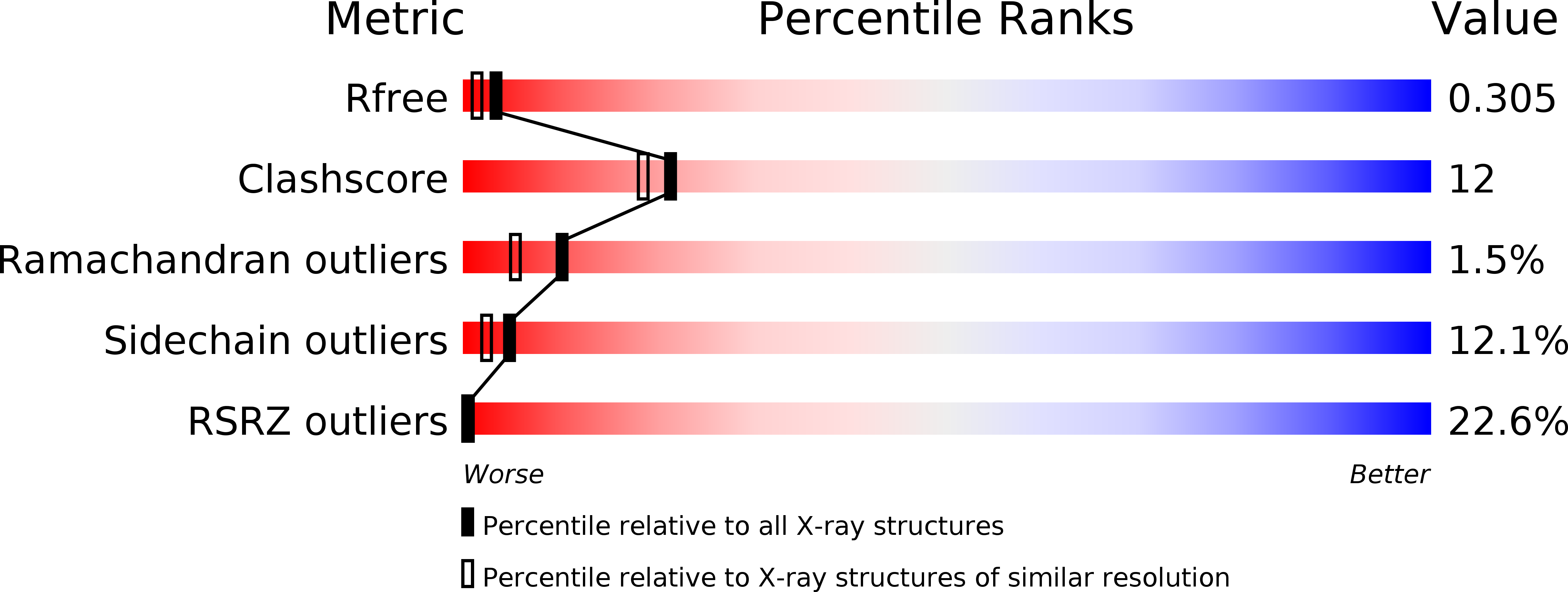
Resolution:
2.09 Å
R-Value Free:
0.27
R-Value Work:
0.27
R-Value Observed:
0.27
Space Group:
C 1 2 1