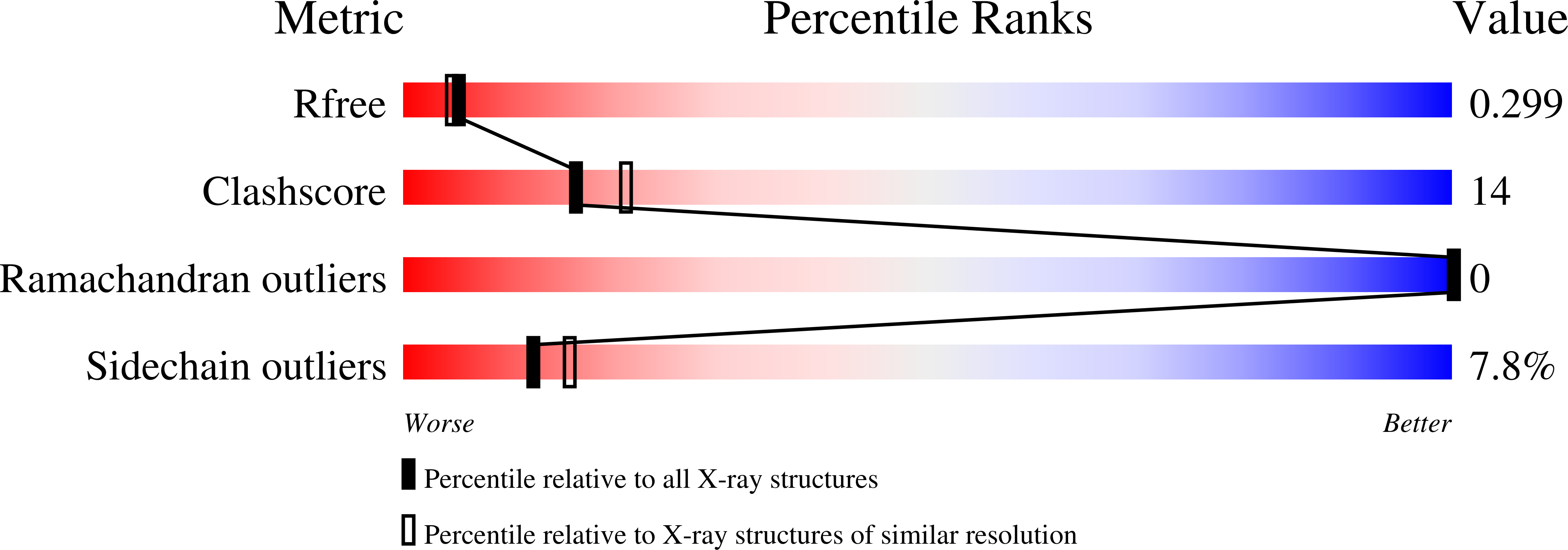
Deposition Date
2008-07-01
Release Date
2008-09-30
Last Version Date
2021-10-20
Entry Detail
PDB ID:
3DMW
Keywords:
Title:
Crystal structure of human type III collagen G982-G1023 containing C-terminal cystine knot
Method Details:
Experimental Method:
Resolution:
2.30 Å
R-Value Free:
0.27
R-Value Work:
0.24
R-Value Observed:
0.24
Space Group:
P 1 21 1