Deposition Date
2008-06-08
Release Date
2009-04-21
Last Version Date
2024-02-21
Entry Detail
PDB ID:
3DE9
Keywords:
Title:
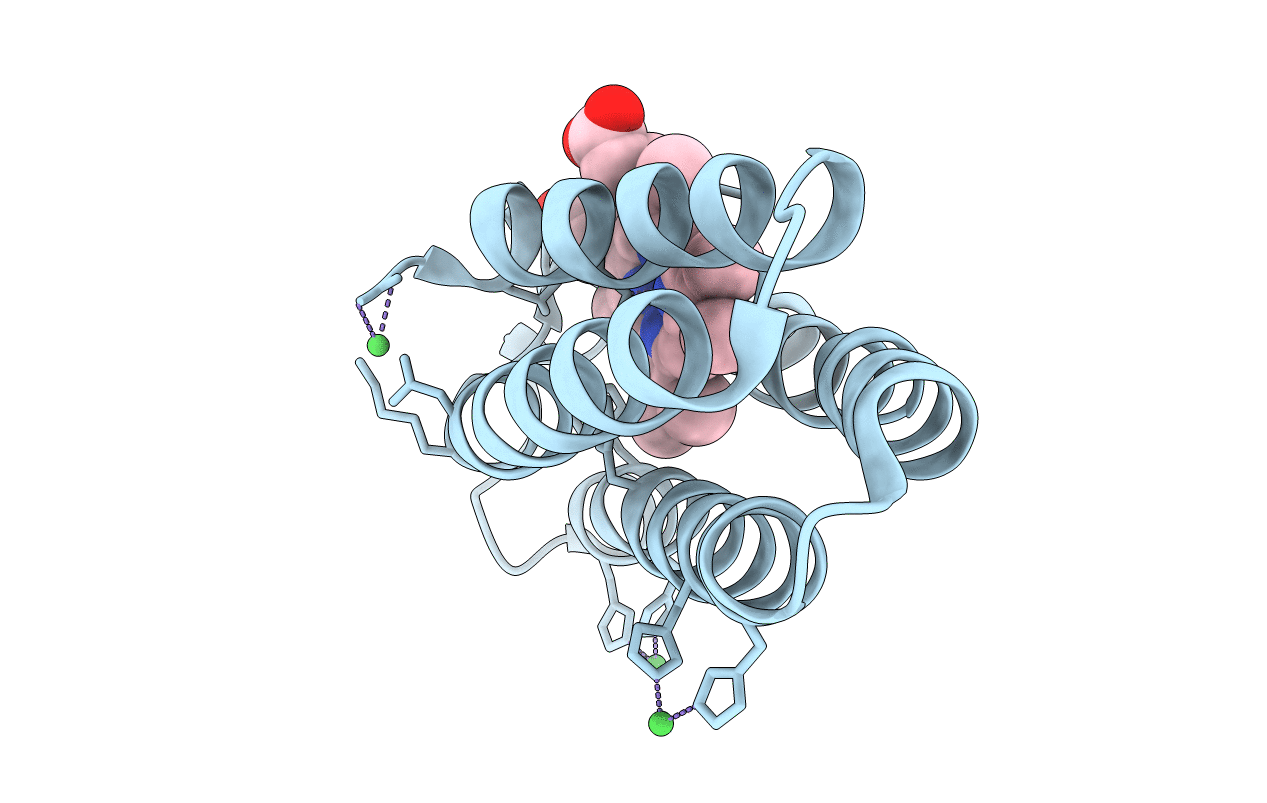
Crystal Structure of a Trimeric Cytochrome cb562 Assembly Induced by Nickel Coordination
Biological Source:
Source Organism:
Escherichia coli (Taxon ID: 562)
Host Organism:
Method Details:
Experimental Method:
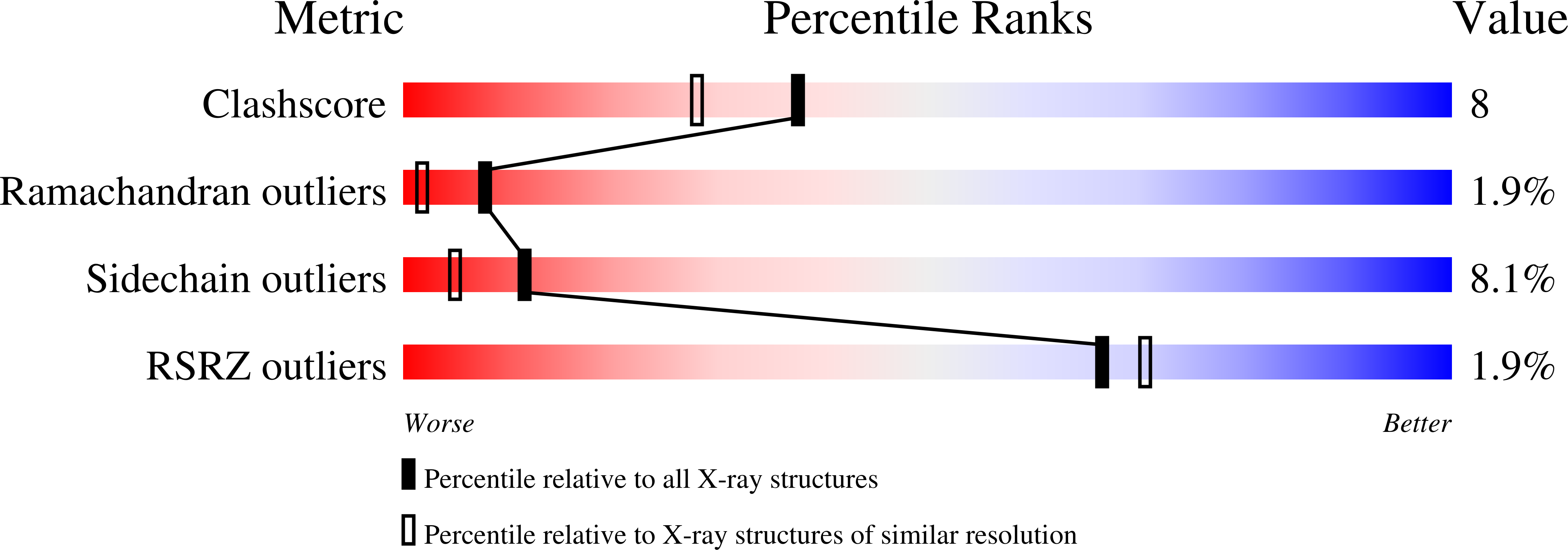
Resolution:
2.04 Å
R-Value Free:
0.27
R-Value Work:
0.21
R-Value Observed:
0.22
Space Group:
H 3