Abstact
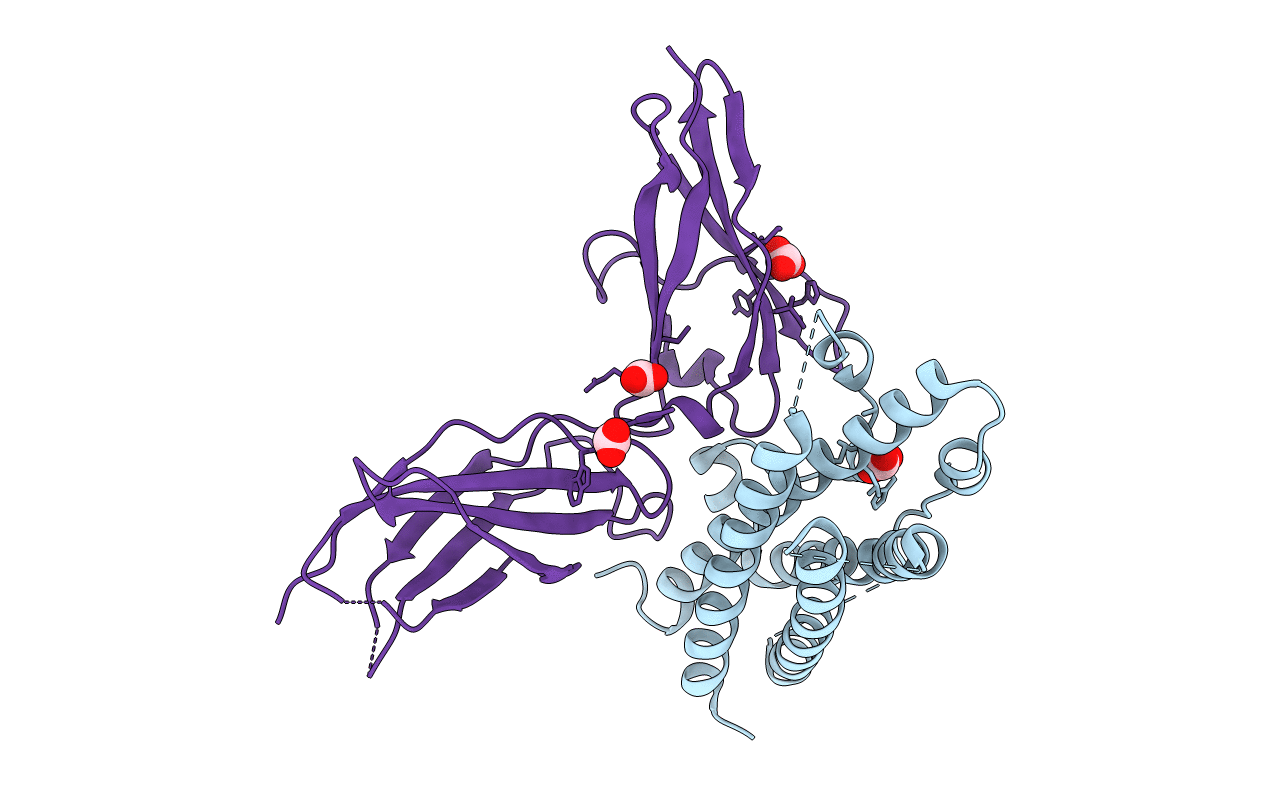
The crystal structure of the complex between an N-terminally truncated G129R human prolactin (PRL) variant and the extracellular domain of the human prolactin receptor (PRLR) was determined at 2.5A resolution by x-ray crystallography. This structure represents the first experimental structure reported for a PRL variant bound to its cognate receptor. The binding of PRL variants to the PRLR extracellular domain was furthermore characterized by the solution state techniques, hydrogen exchange mass spectrometry, and NMR spectroscopy. Compared with the binding interface derived from mutagenesis studies, the structural data imply that the definition of PRL binding site 1 should be extended to include residues situated in the N-terminal part of loop 1 and in the C terminus. Comparison of the structure of the receptor-bound PRL variant with the structure reported for the unbound form of a similar analogue (Jomain, J. B., Tallet, E., Broutin, I., Hoos, S., van Agthoven, J., Ducruix, A., Kelly, P. A., Kragelund, B. B., England, P., and Goffin, V. (2007) J. Biol. Chem. 282, 33118-33131) demonstrates that receptor-induced changes in the backbone of the four-helix bundle are subtle, whereas large scale rearrangements and structuring occur in the flexible N-terminal part of loop 1. Hydrogen exchange mass spectrometry data imply that the dynamics of the four-helix bundle in solution generally become stabilized upon receptor interaction at binding site 1.