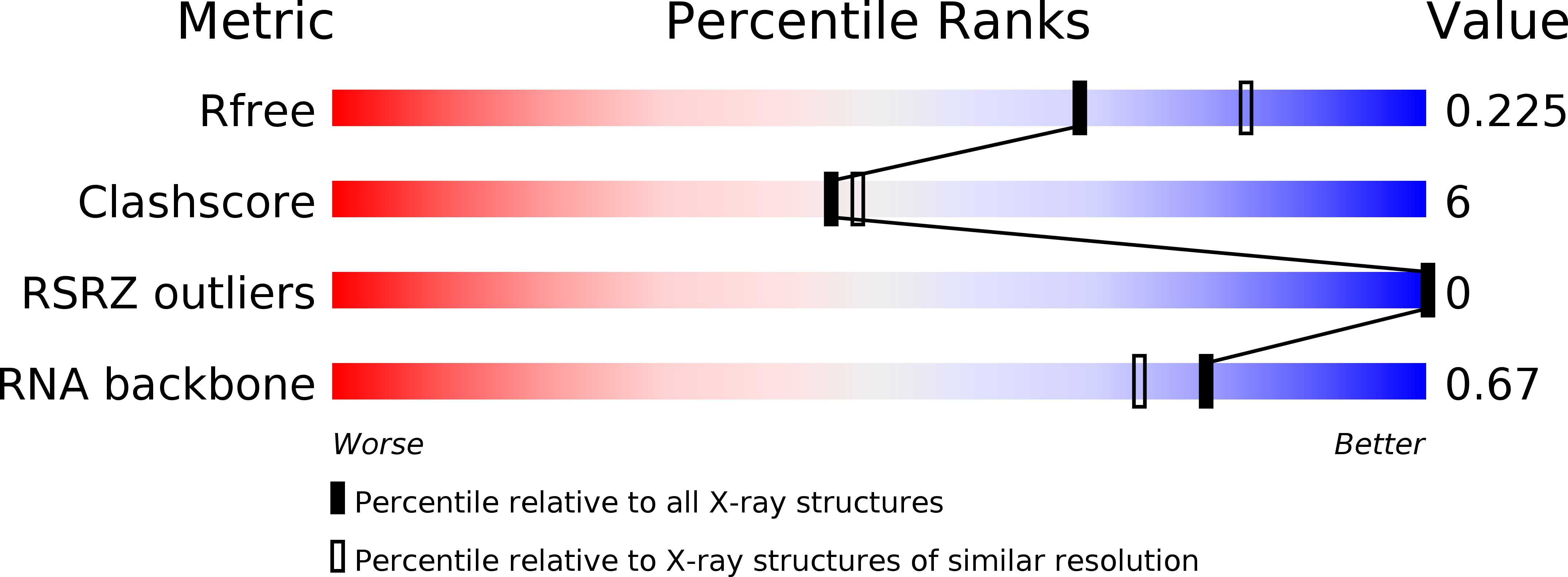
Deposition Date
2008-04-04
Release Date
2008-08-26
Last Version Date
2023-08-30
Entry Detail
PDB ID:
3CR1
Keywords:
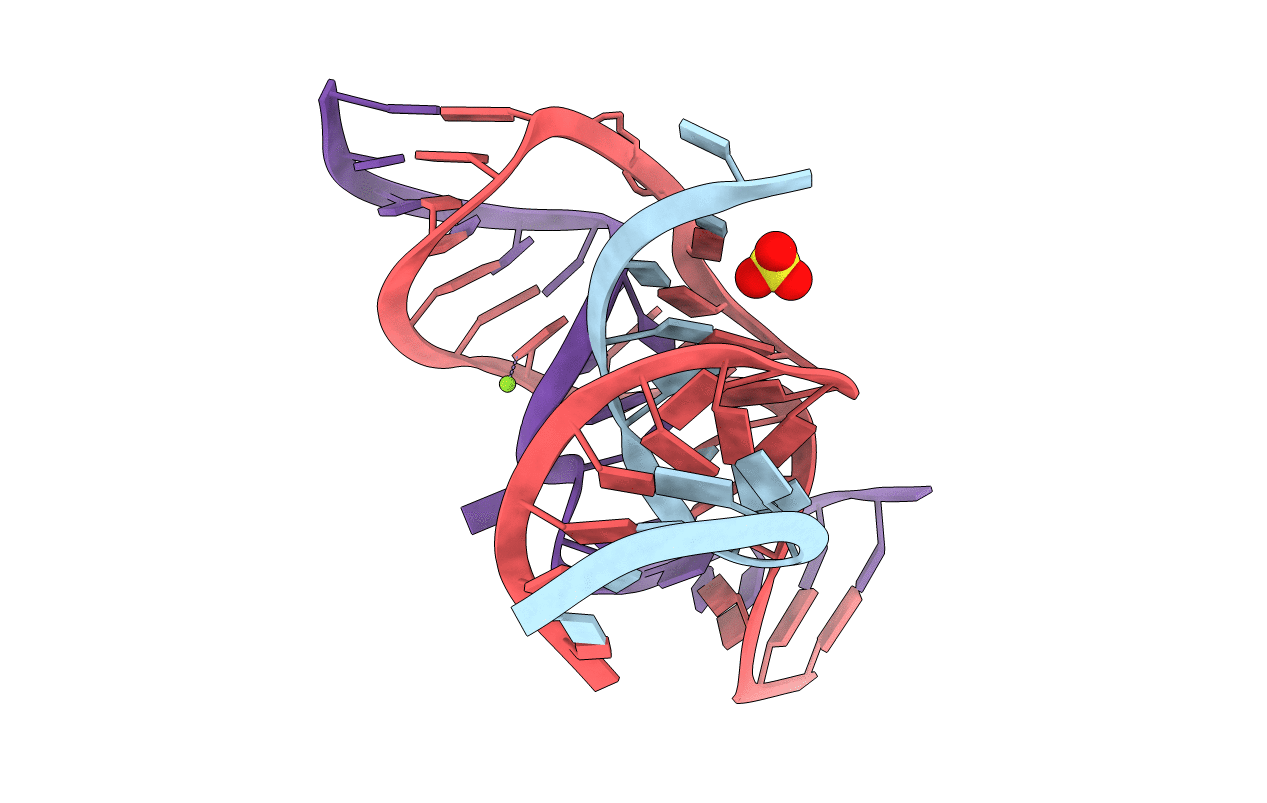
Title:
crystal structure of a minimal, mutant, all-RNA hairpin ribozyme (A38C, A-1OMA) grown from MgCl2
Biological Source:
Source Organism:
Method Details:
Experimental Method:
Resolution:
2.25 Å
R-Value Free:
0.24
R-Value Work:
0.21
Space Group:
P 61 2 2