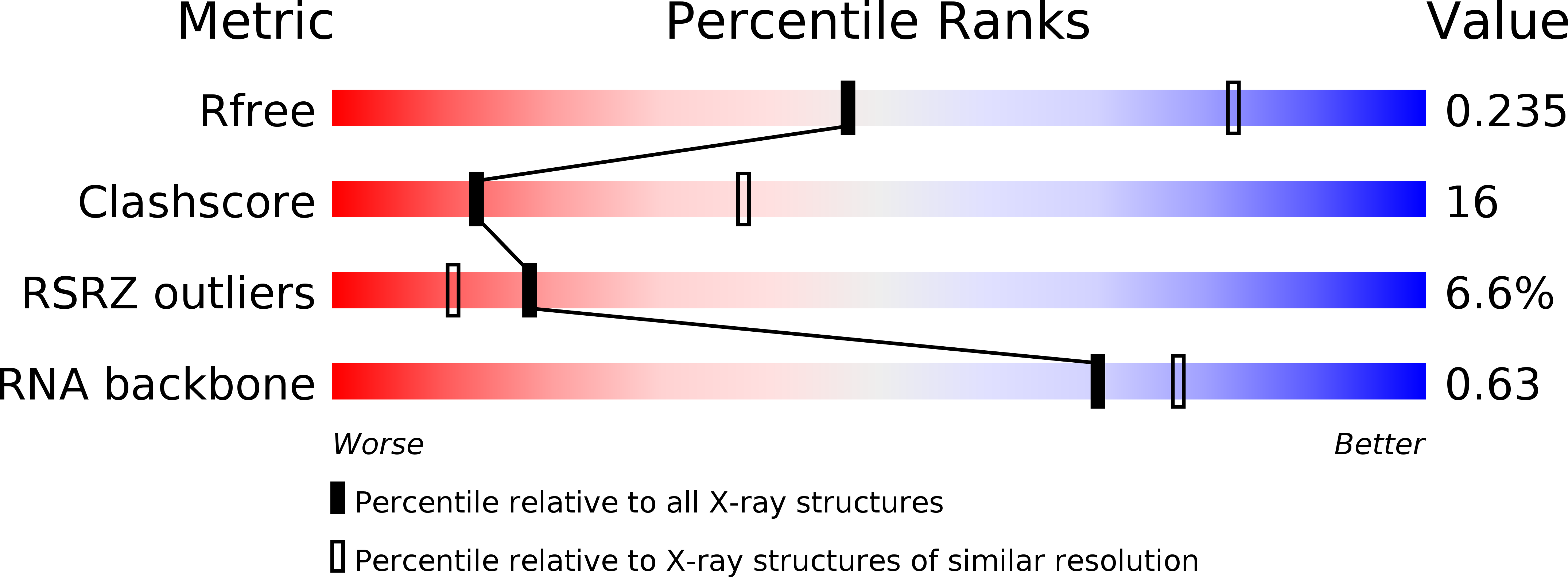
Deposition Date
2008-04-03
Release Date
2008-05-20
Last Version Date
2023-08-30
Entry Detail
PDB ID:
3CQS
Keywords:
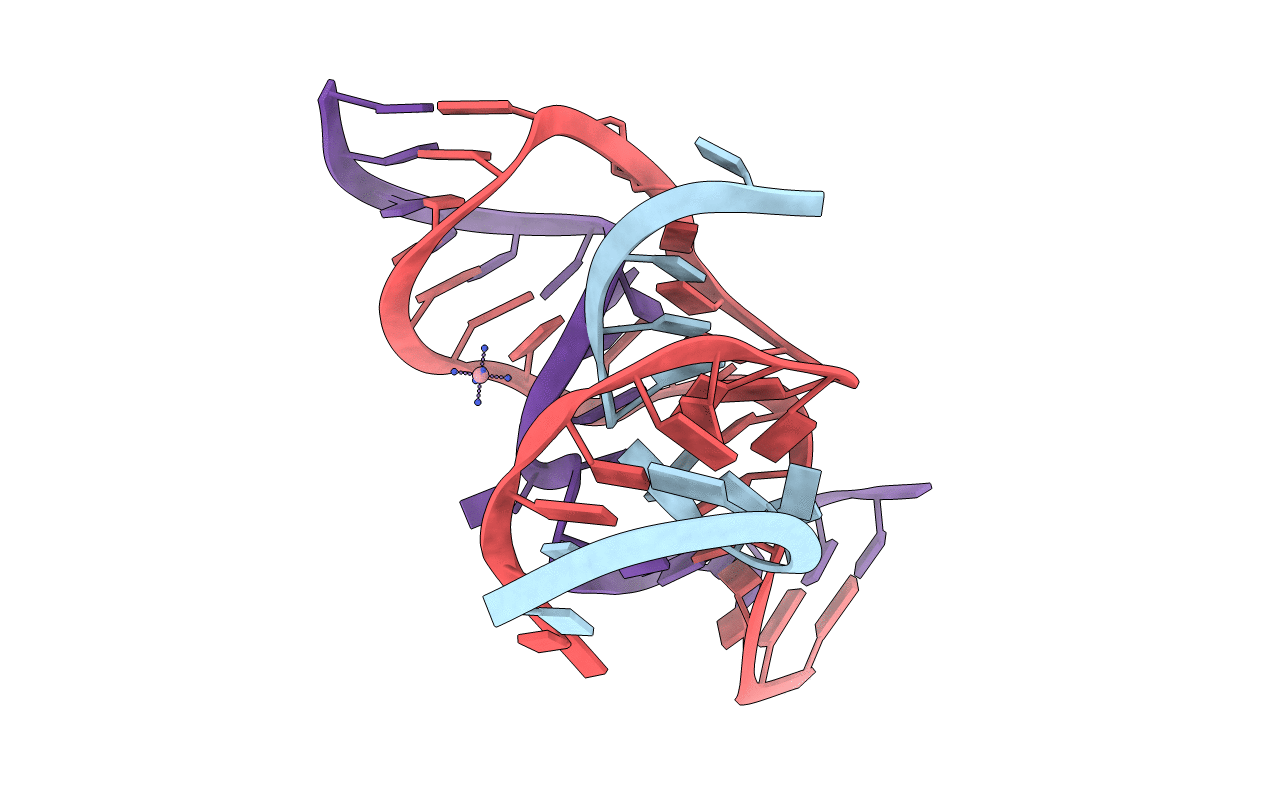
Title:
A 3'-OH, 2',5'-phosphodiester substitution in the hairpin ribozyme active site reveals similarities with protein ribonucleases
Method Details:
Experimental Method:
Resolution:
2.80 Å
R-Value Free:
0.25
R-Value Work:
0.21
R-Value Observed:
0.22
Space Group:
P 61 2 2