Deposition Date
2008-03-25
Release Date
2009-03-10
Last Version Date
2024-11-13
Entry Detail
PDB ID:
3CN7
Keywords:
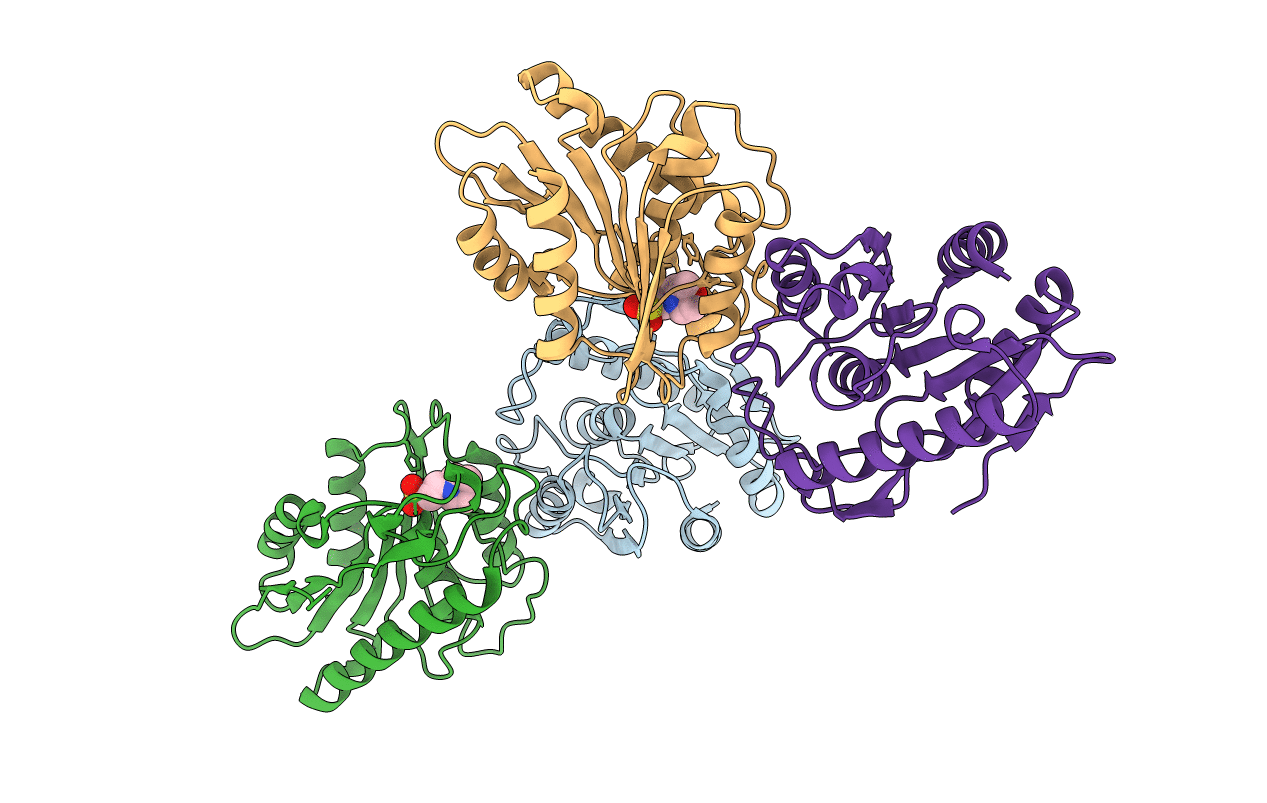
Title:
Crystal Structure Analysis of the Carboxylesterase PA3859 from Pseudomonas aeruginosa PAO1- MONOCLINIC CRYSTAL FORM
Biological Source:
Source Organism:
Pseudomonas aeruginosa (Taxon ID: 287)
Host Organism:
Method Details:
Experimental Method:
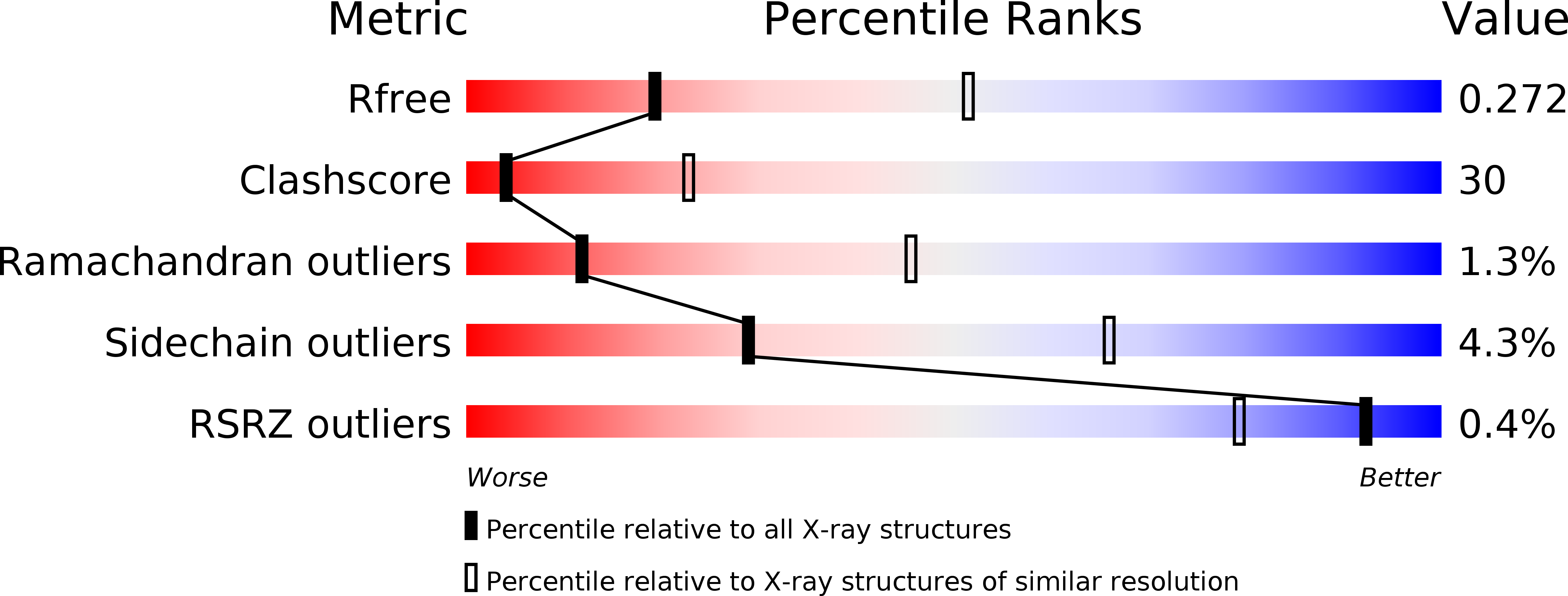
Resolution:
2.99 Å
R-Value Free:
0.27
R-Value Work:
0.20
R-Value Observed:
0.20
Space Group:
P 1 21 1