Deposition Date
2008-02-15
Release Date
2008-03-11
Last Version Date
2024-04-03
Entry Detail
PDB ID:
3C9J
Keywords:
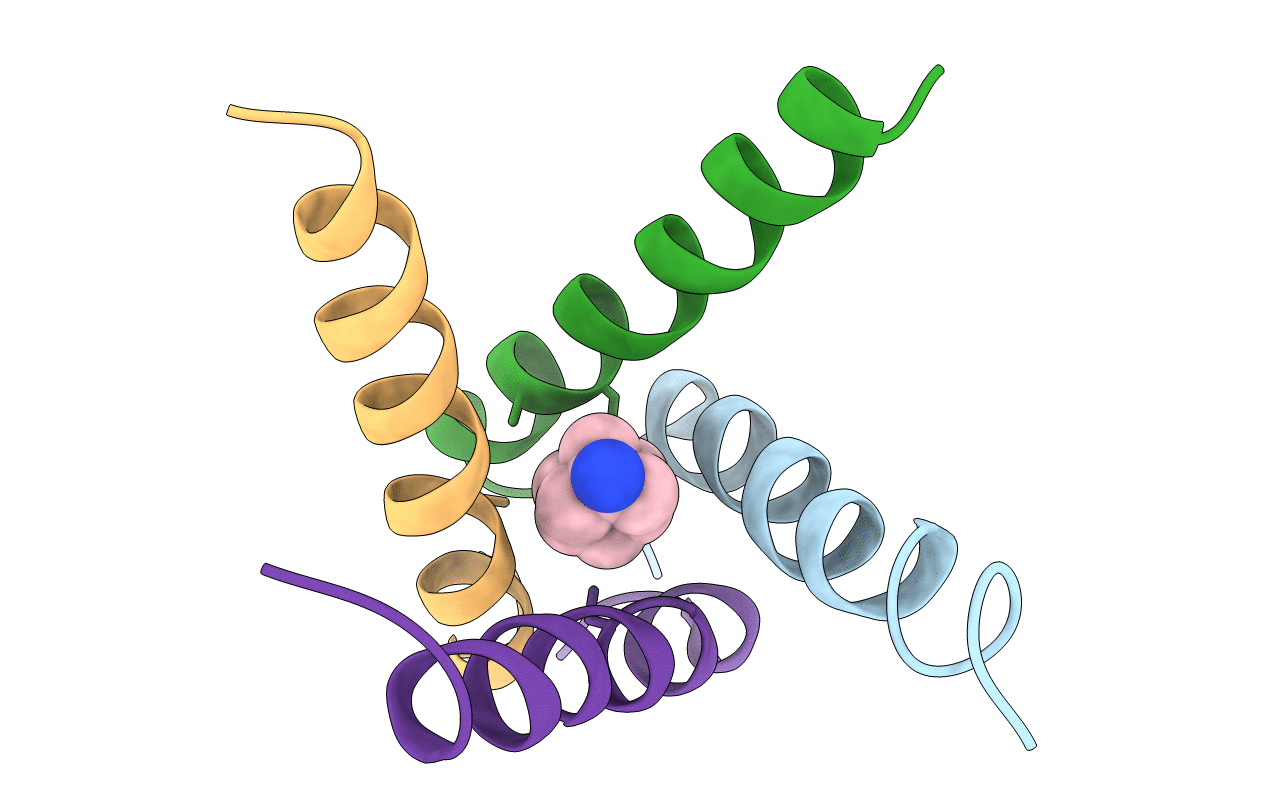
Title:
The Crystal structure of Transmembrane domain of M2 protein and Amantadine complex
Method Details:
Experimental Method:
Resolution:
3.50 Å
R-Value Free:
0.31
R-Value Work:
0.29
R-Value Observed:
0.29
Space Group:
P 21 21 2


