Deposition Date
2007-12-14
Release Date
2008-06-24
Last Version Date
2024-02-21
Entry Detail
PDB ID:
3BNR
Keywords:
Title:
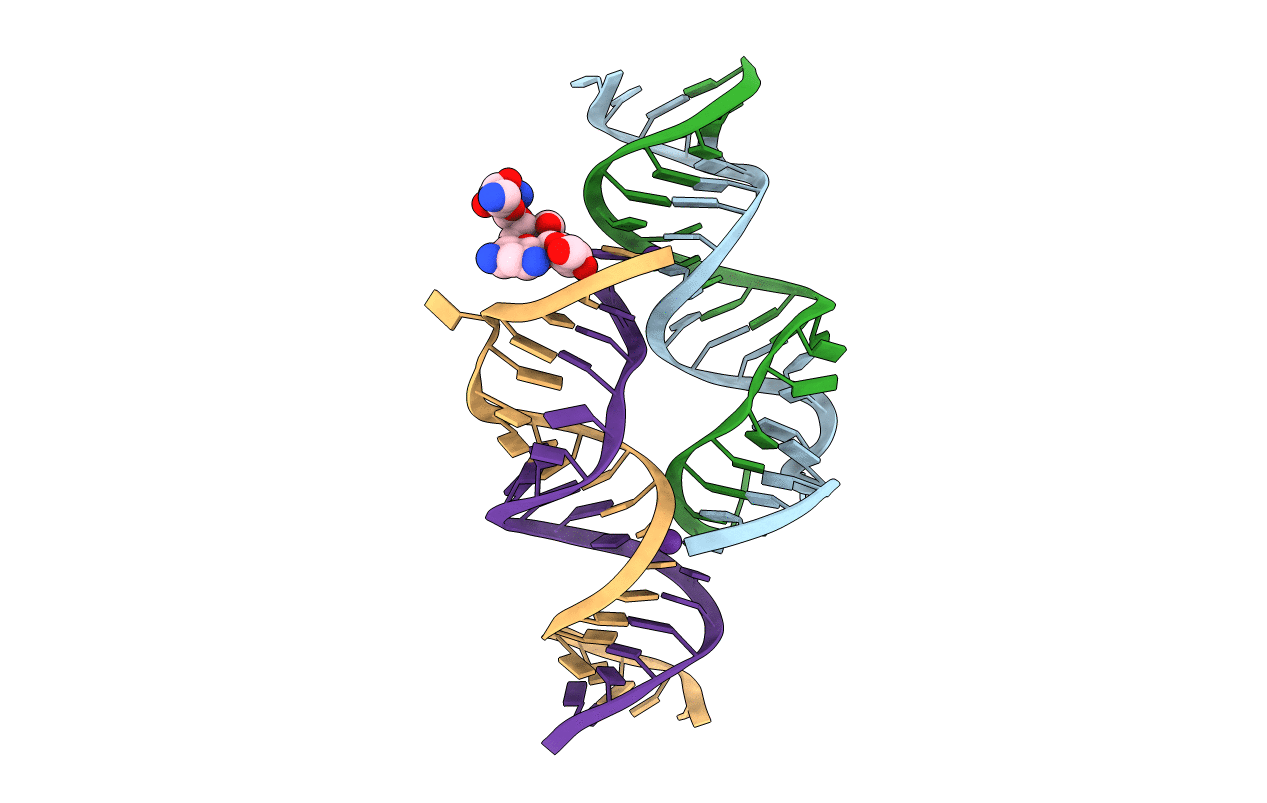
Crystal Structure of the Homo sapiens Mitochondrial Ribosomal Decoding Site in the presence of nonspecifically bound paromomycin (A1555G mutant, Br-derivative)
Method Details:
Experimental Method:
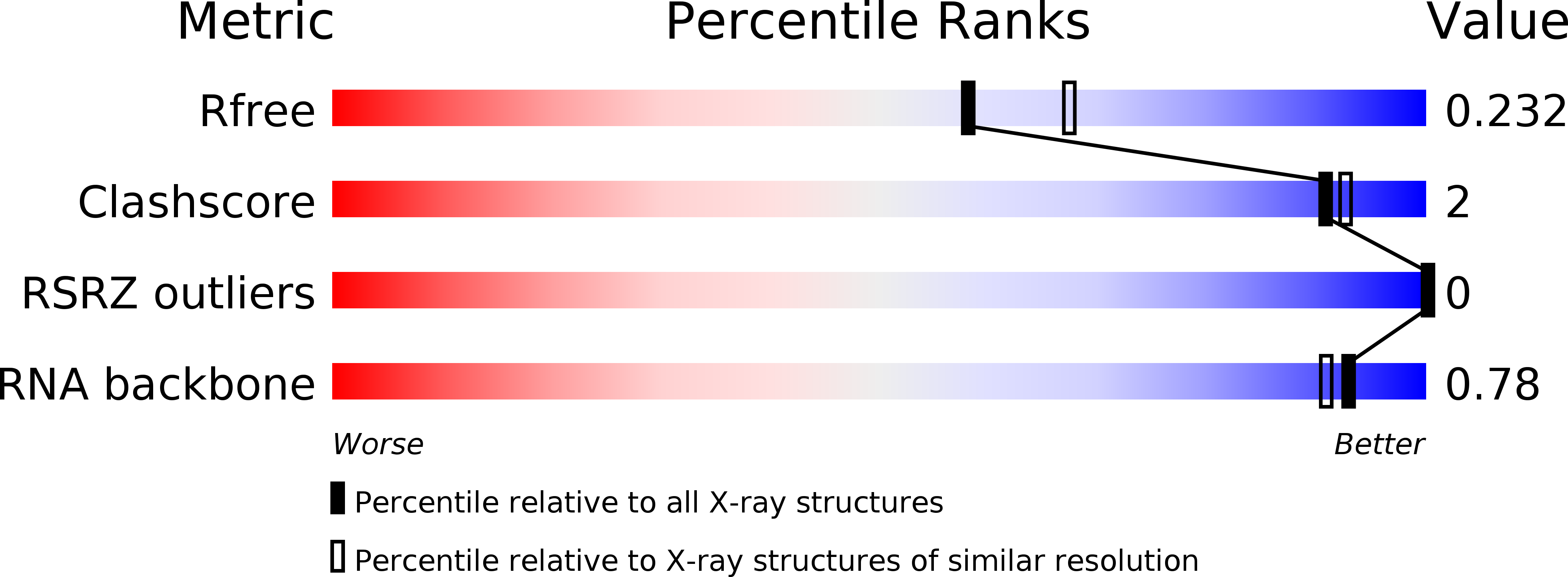
Resolution:
2.10 Å
R-Value Free:
0.24
R-Value Work:
0.21
Space Group:
C 1 2 1