Deposition Date
1999-03-08
Release Date
1999-04-26
Last Version Date
2023-12-27
Entry Detail
PDB ID:
3BDO
Keywords:
Title:
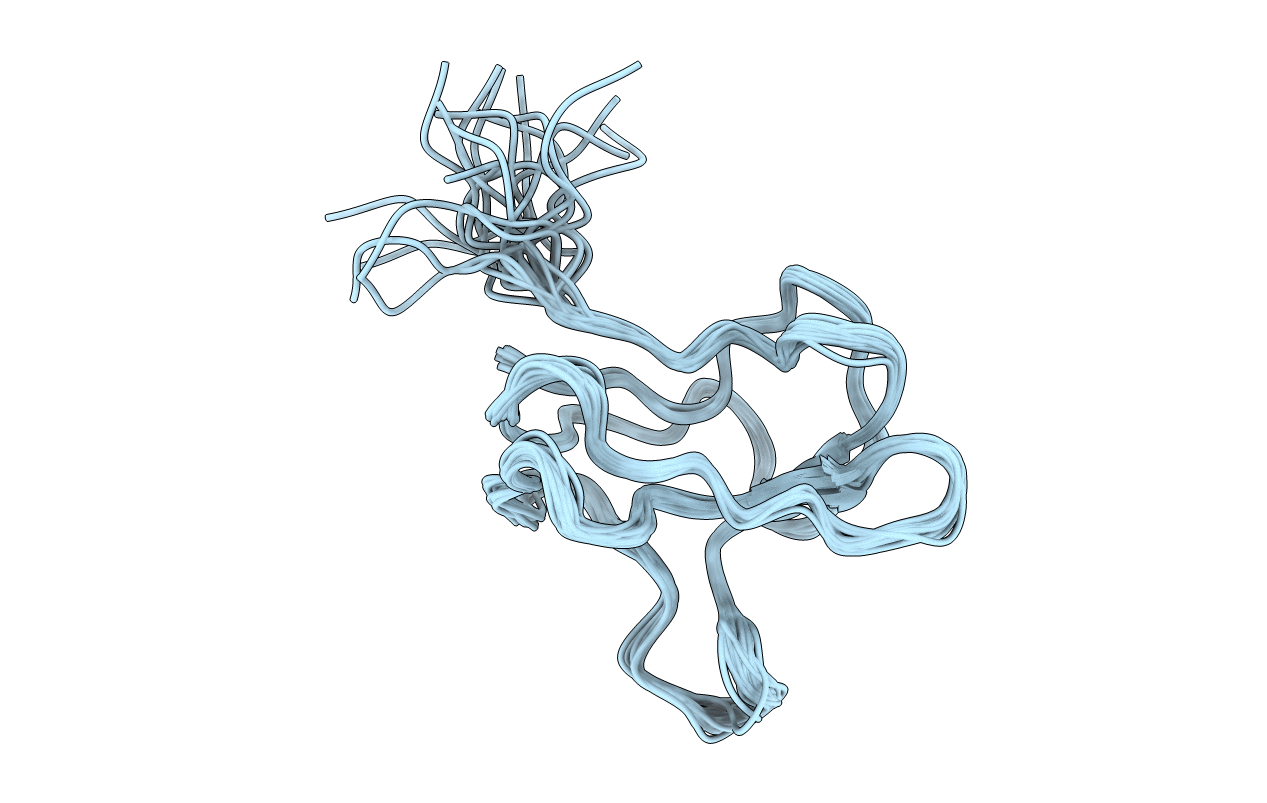
SOLUTION STRUCTURE OF APO-BIOTINYL DOMAIN FROM ACETYL COENZYME A CARBOXYLASE OF ESCHERICHIA COLI DETERMINED BY TRIPLE-RESONANCE NMR SPECTROSCOPY
Biological Source:
Source Organism(s):
Escherichia coli (Taxon ID: 469008)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
40
Conformers Submitted:
20
Selection Criteria:
LOWEST ENERGY


