Deposition Date
2007-11-09
Release Date
2008-08-12
Last Version Date
2023-08-30
Entry Detail
PDB ID:
3BBK
Keywords:
Title:
Miminally Junctioned Hairpin Ribozyme Incorporates A38C and 2'5'-phosphodiester Linkage within Active Site
Method Details:
Experimental Method:
Resolution:
2.75 Å
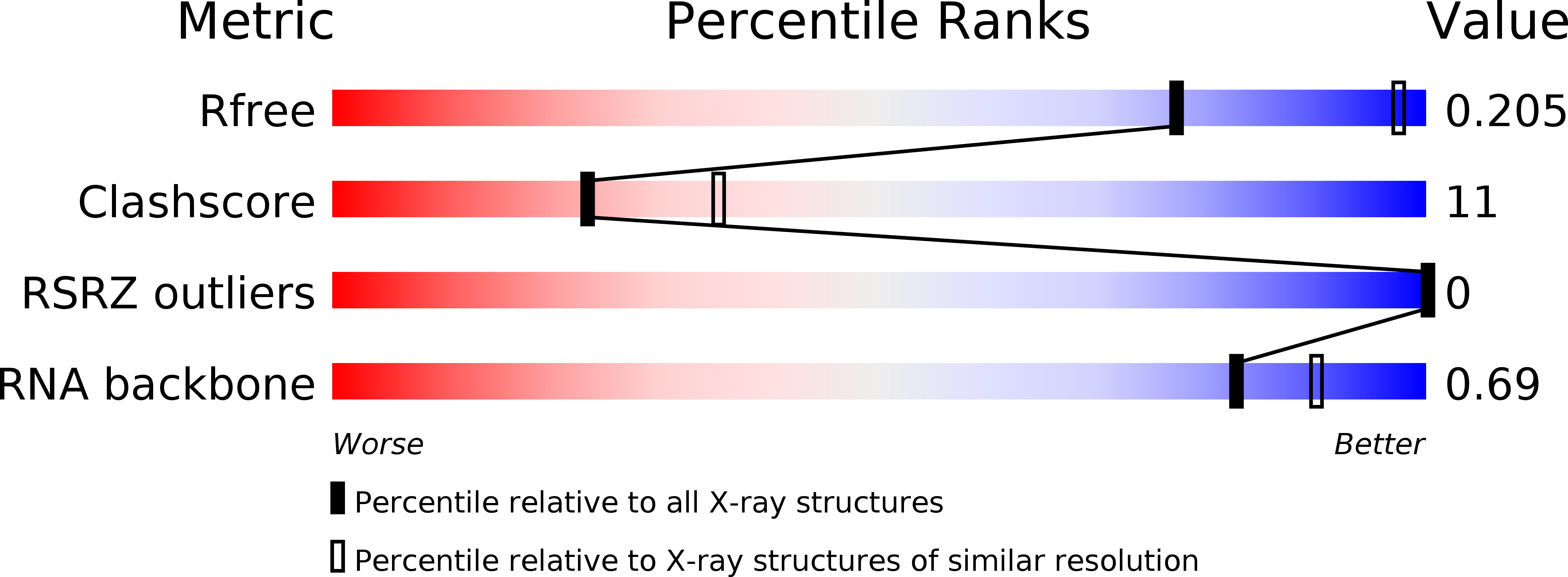
R-Value Free:
0.22
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 61 2 2