Deposition Date
2011-03-28
Release Date
2011-07-06
Last Version Date
2024-10-23
Entry Detail
PDB ID:
3AX3
Keywords:
Title:
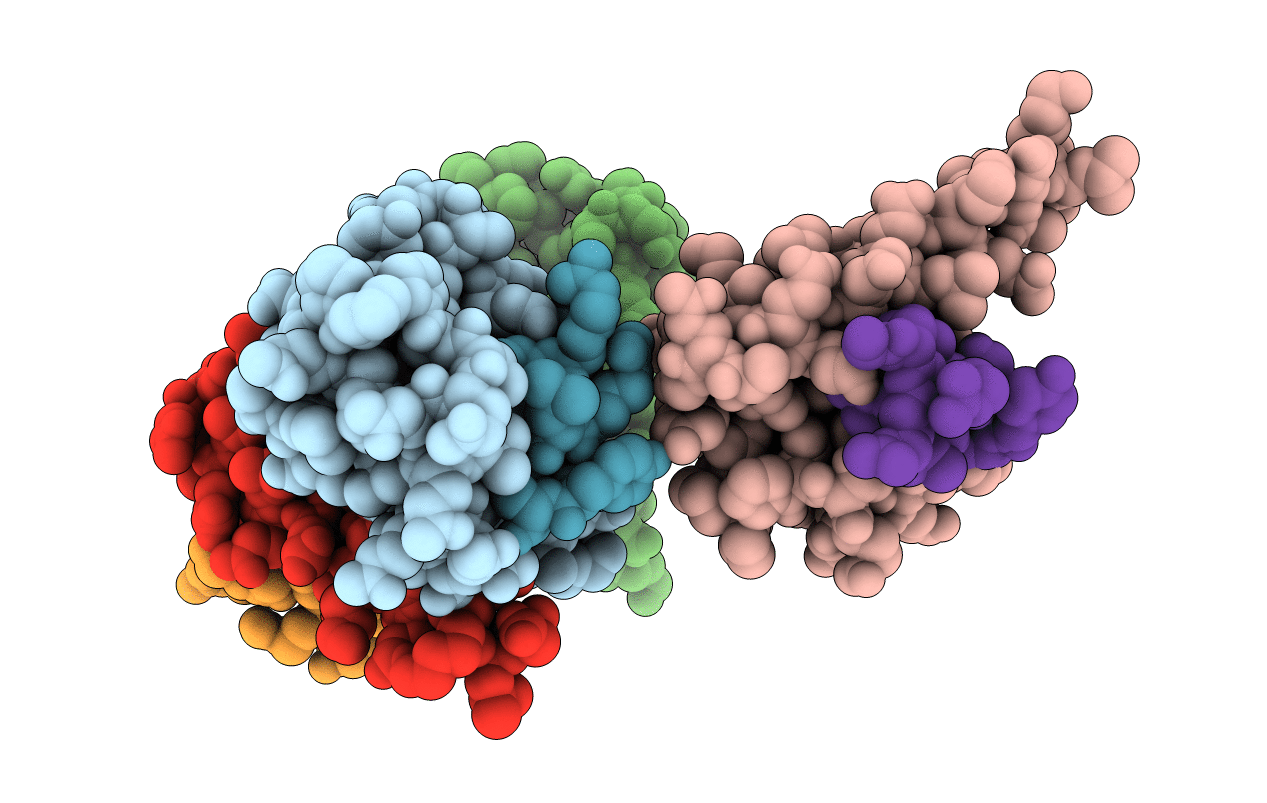
Crystal structure of rat TOM20-ALDH presequence complex: a complex (form2) between Tom20 and a disulfide-bridged presequence peptide containing D-Cys and L-Cys at the i and i+3 positions.
Biological Source:
Source Organism(s):
Rattus norvegicus (Taxon ID: 10116)
Expression System(s):
Method Details:
Experimental Method:
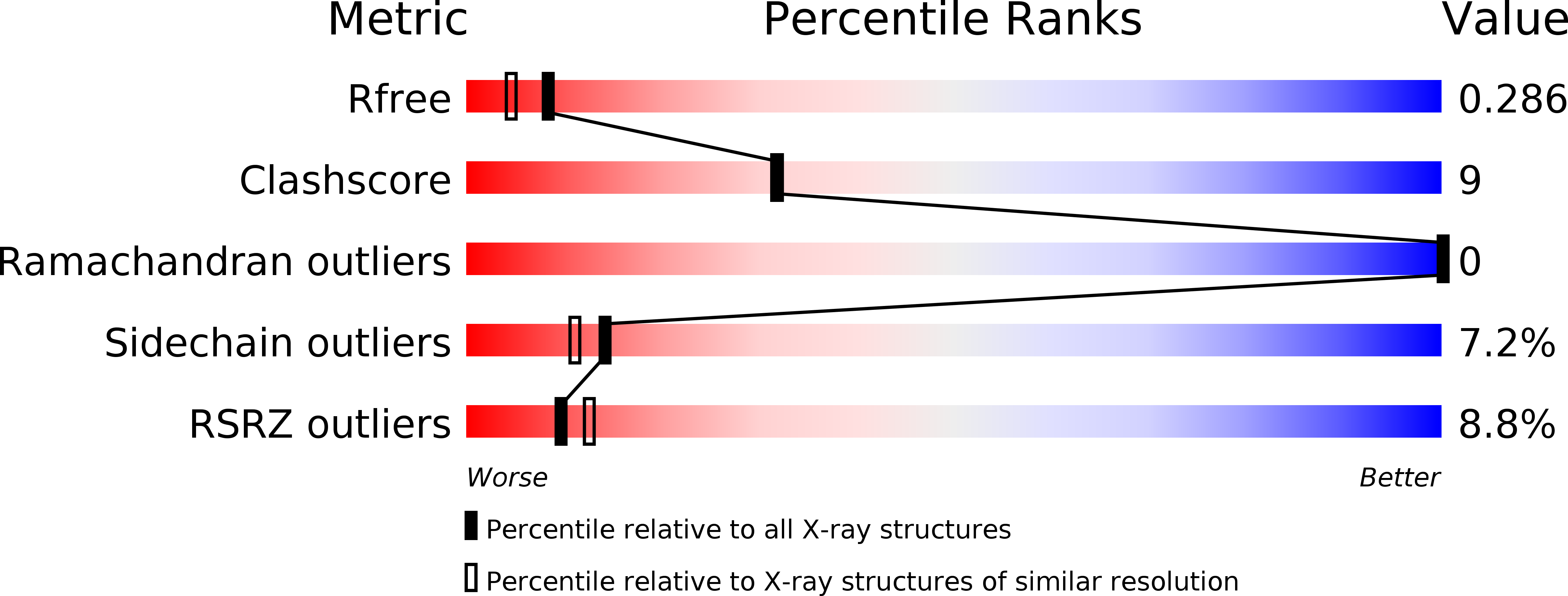
Resolution:
2.10 Å
R-Value Free:
0.28
R-Value Work:
0.24
R-Value Observed:
0.24
Space Group:
H 3 2