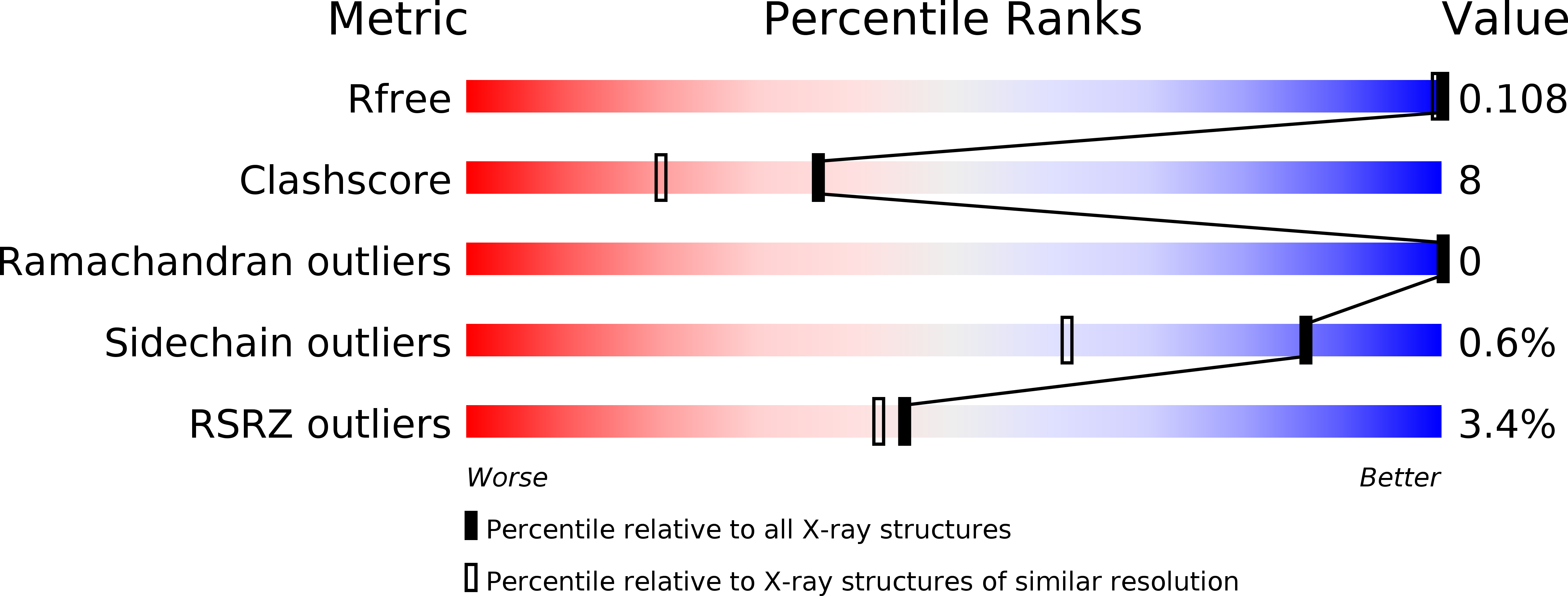
Deposition Date
2010-07-29
Release Date
2011-06-08
Last Version Date
2024-11-20
Entry Detail
PDB ID:
3ALD
Keywords:
Title:
Crystal structure of sweet-tasting protein Thaumatin I at 1.10 A
Biological Source:
Source Organism(s):
Thaumatococcus daniellii (Taxon ID: 4621)
Method Details: