Deposition Date
2010-06-07
Release Date
2010-12-22
Last Version Date
2023-11-15
Entry Detail
PDB ID:
3AJI
Keywords:
Title:
Structure of Gankyrin-S6ATPase photo-cross-linked site-specifically, and incoporated by genetic code expansion
Biological Source:
Source Organism:
Mus musculus (Taxon ID: 10090)
Host Organism:
Method Details:
Experimental Method:
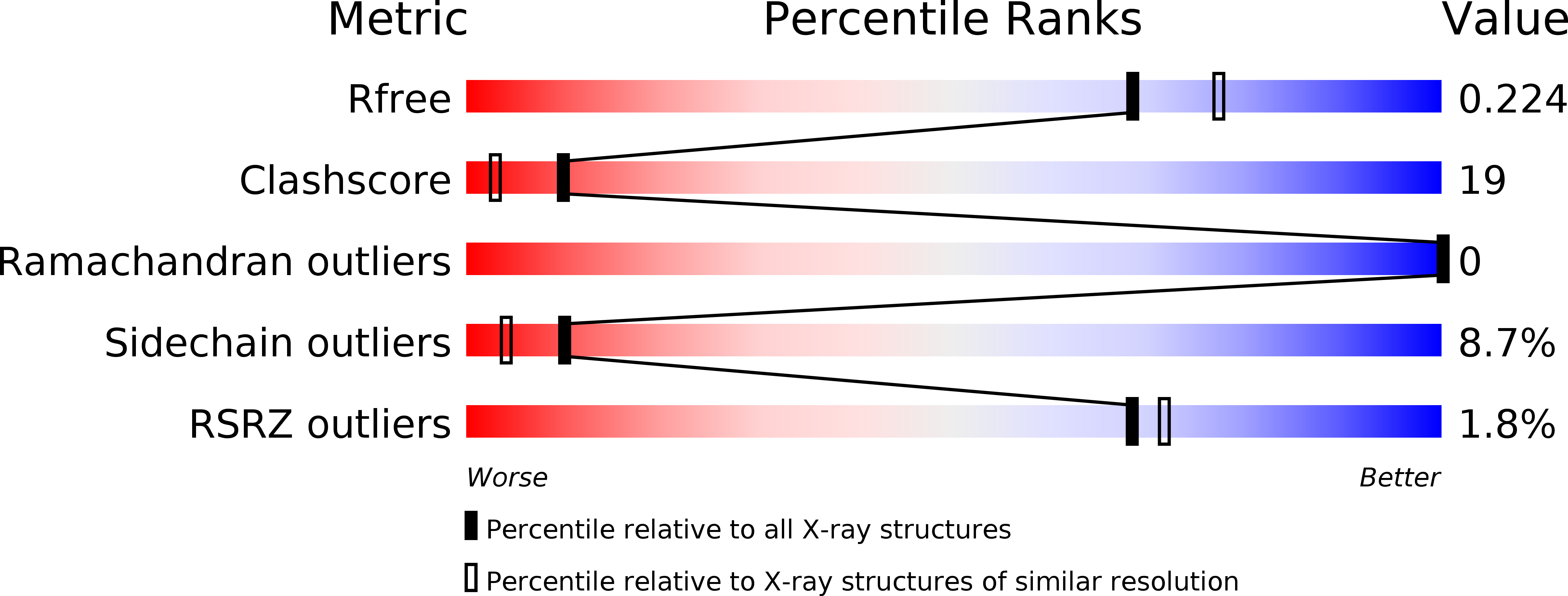
Resolution:
2.05 Å
R-Value Free:
0.22
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
H 3