Deposition Date
2009-12-16
Release Date
2010-12-01
Last Version Date
2025-03-26
Entry Detail
PDB ID:
3ABN
Keywords:
Title:
Crystal structure of (Pro-Pro-Gly)4-Hyp-Asp-Gly-(Pro-Pro-Gly)4 at 1.02 A
Method Details:
Experimental Method:
Resolution:
1.02 Å
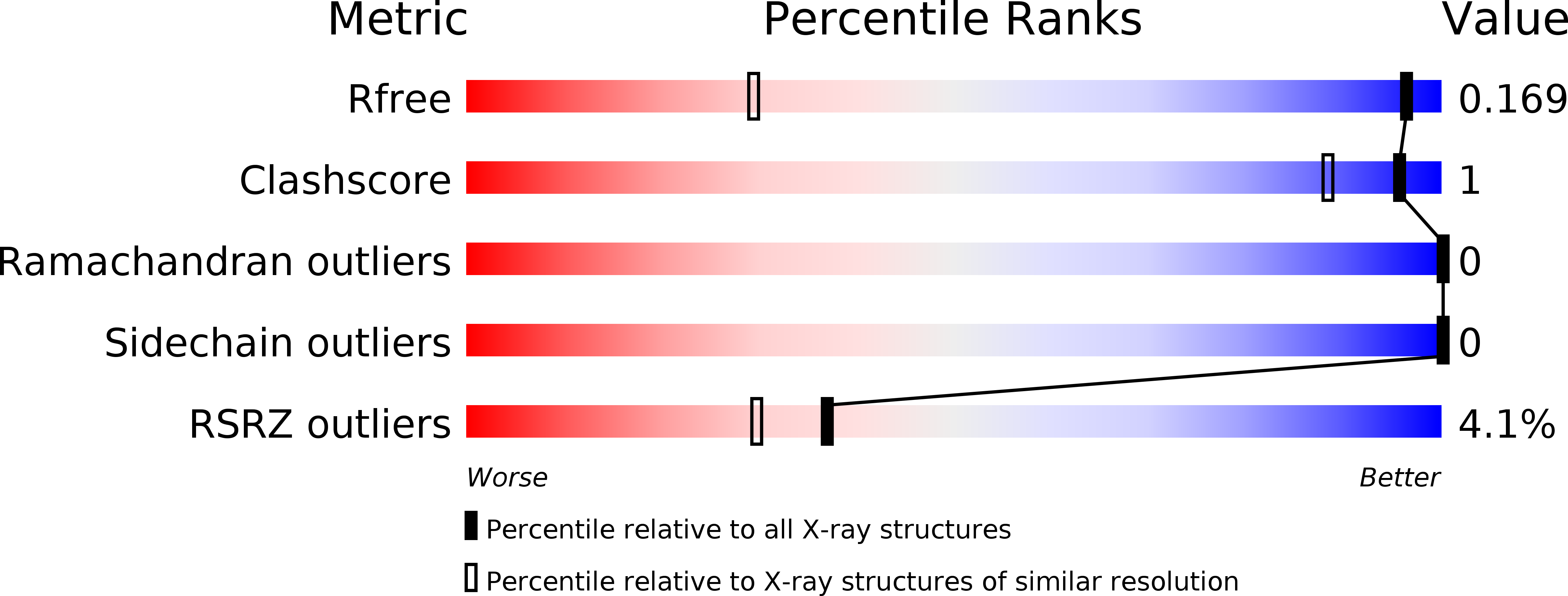
R-Value Free:
0.16
R-Value Work:
0.13
Space Group:
P 1 21 1