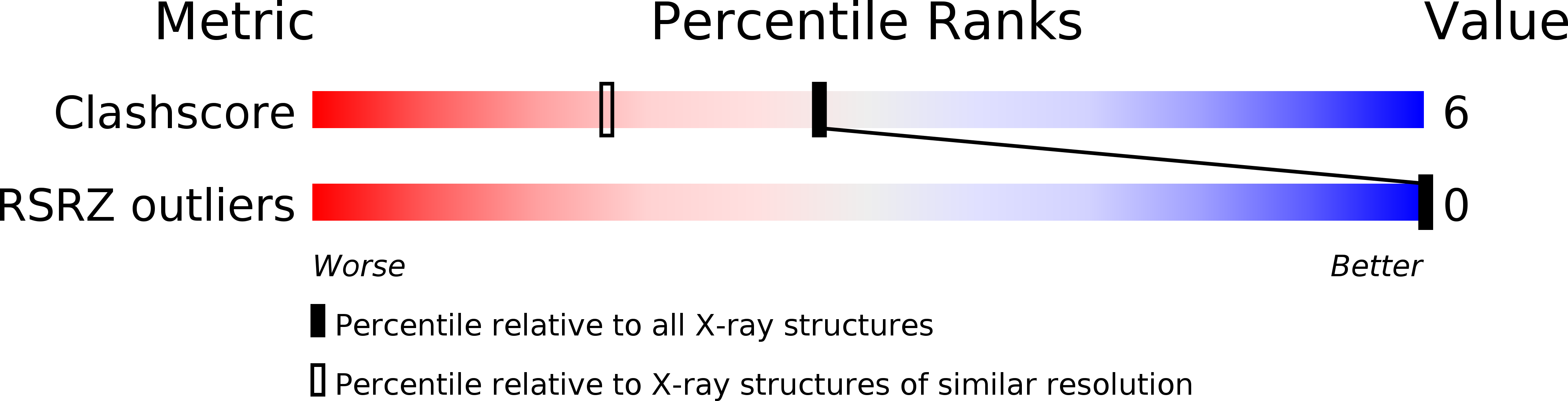
Deposition Date
1997-10-07
Release Date
1997-10-13
Last Version Date
2023-08-02
Method Details:
Experimental Method:
Resolution:
1.40 Å
R-Value Free:
0.21
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 21 21 21