Deposition Date
2006-09-19
Release Date
2007-05-01
Last Version Date
2024-10-30
Entry Detail
PDB ID:
2IEZ
Keywords:
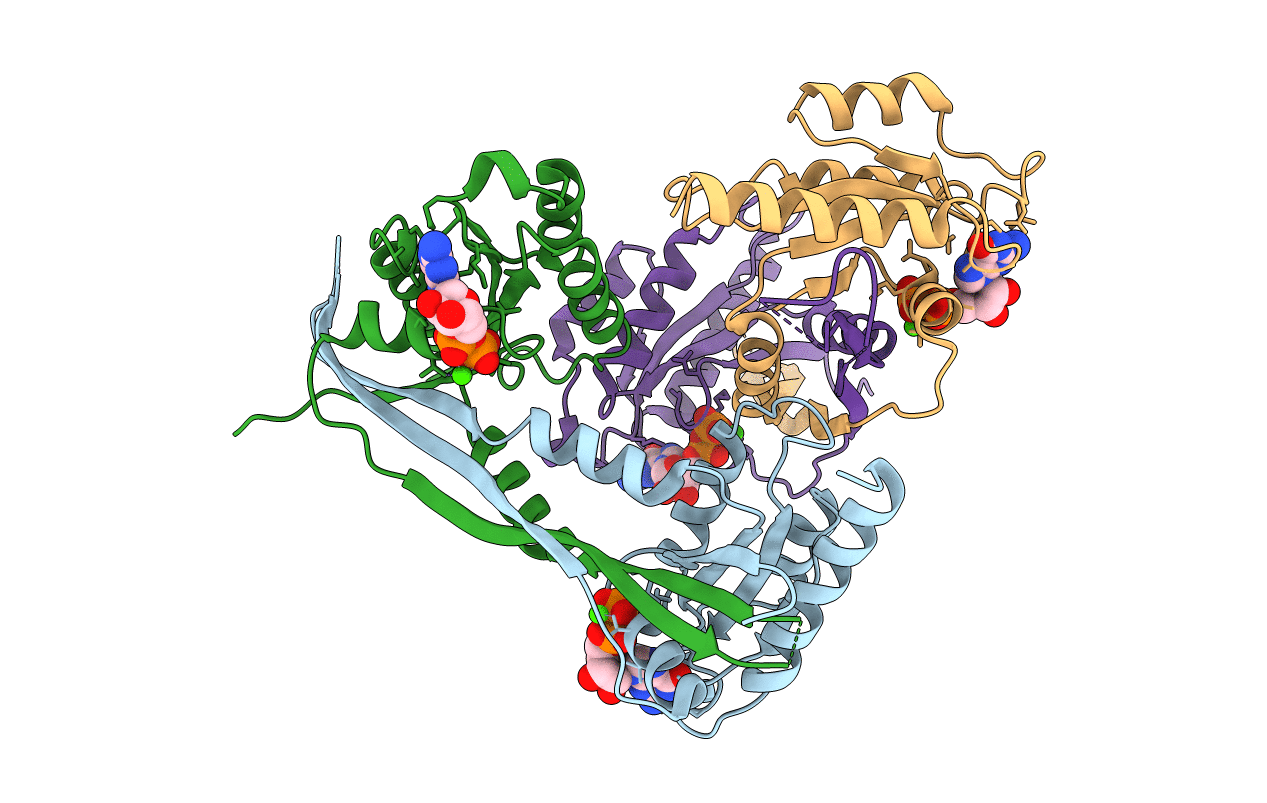
Title:
Crystal Structure of mouse Rab27b bound to GDP in monoclinic space group
Biological Source:
Source Organism:
Mus musculus (Taxon ID: 10090)
Host Organism:
Method Details:
Experimental Method:
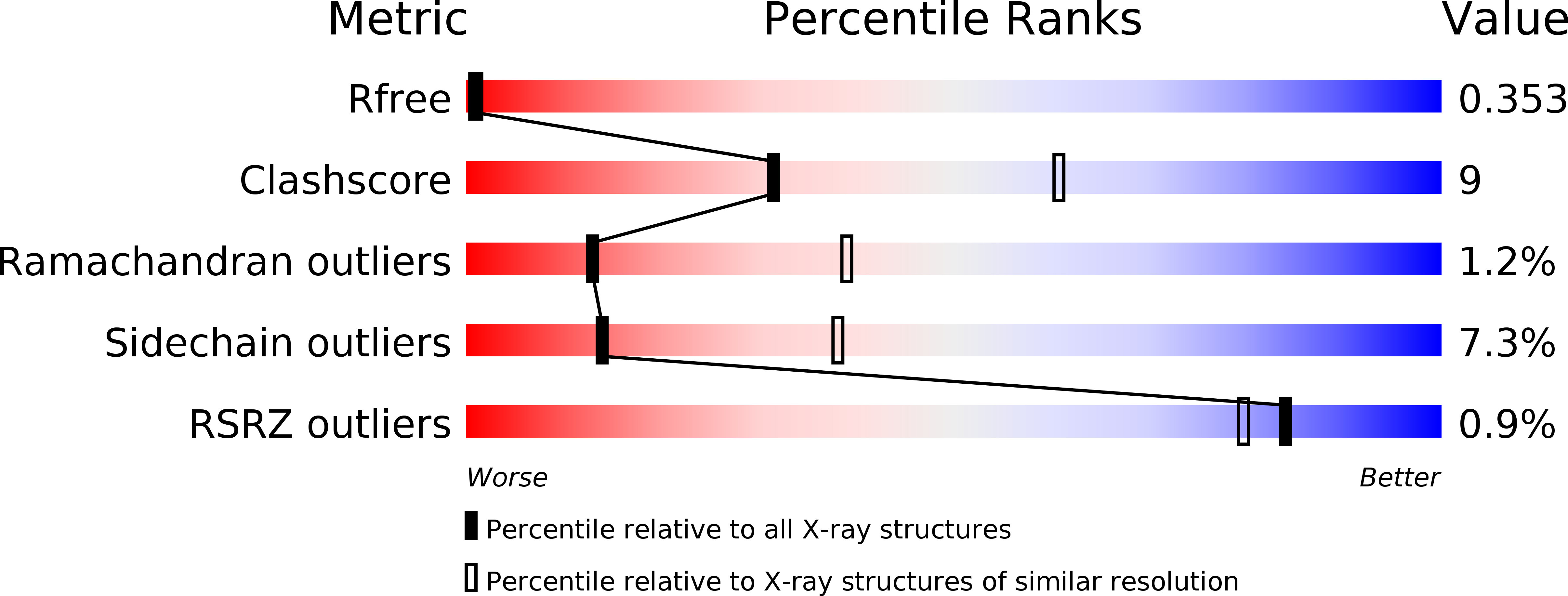
Resolution:
2.80 Å
R-Value Free:
0.36
R-Value Work:
0.26
R-Value Observed:
0.26
Space Group:
C 1 2 1