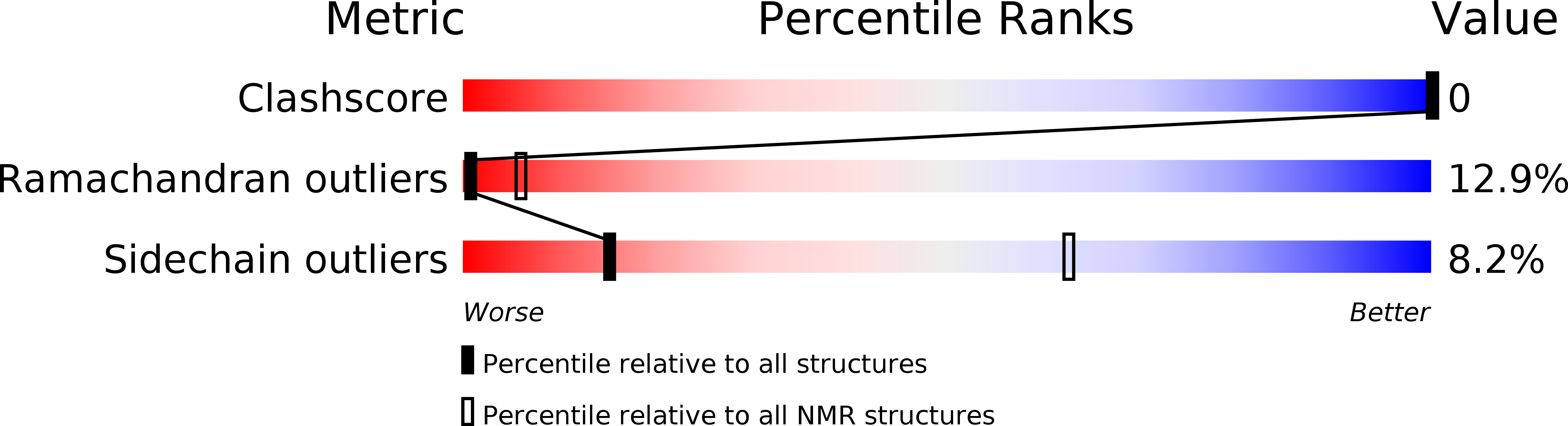Deposition Date
1993-04-13
Release Date
1993-10-31
Last Version Date
2024-10-16
Entry Detail
PDB ID:
2ECH
Keywords:
Title:
ECHISTATIN-THE REFINED STRUCTURE OF A DISINTEGRIN IN SOLUTION BY 1H NMR
Biological Source:
Source Organism(s):
Echis carinatus (Taxon ID: 40353)
Method Details:
Experimental Method:
Conformers Submitted:
8