Deposition Date
2009-02-20
Release Date
2009-08-04
Last Version Date
2024-10-16
Entry Detail
PDB ID:
2ZZO
Keywords:
Title:
Crystal structure of the complex between GP41 fragment N36 and fusion inhibitor C34/S138A
Biological Source:
Source Organism:
Method Details:
Experimental Method:
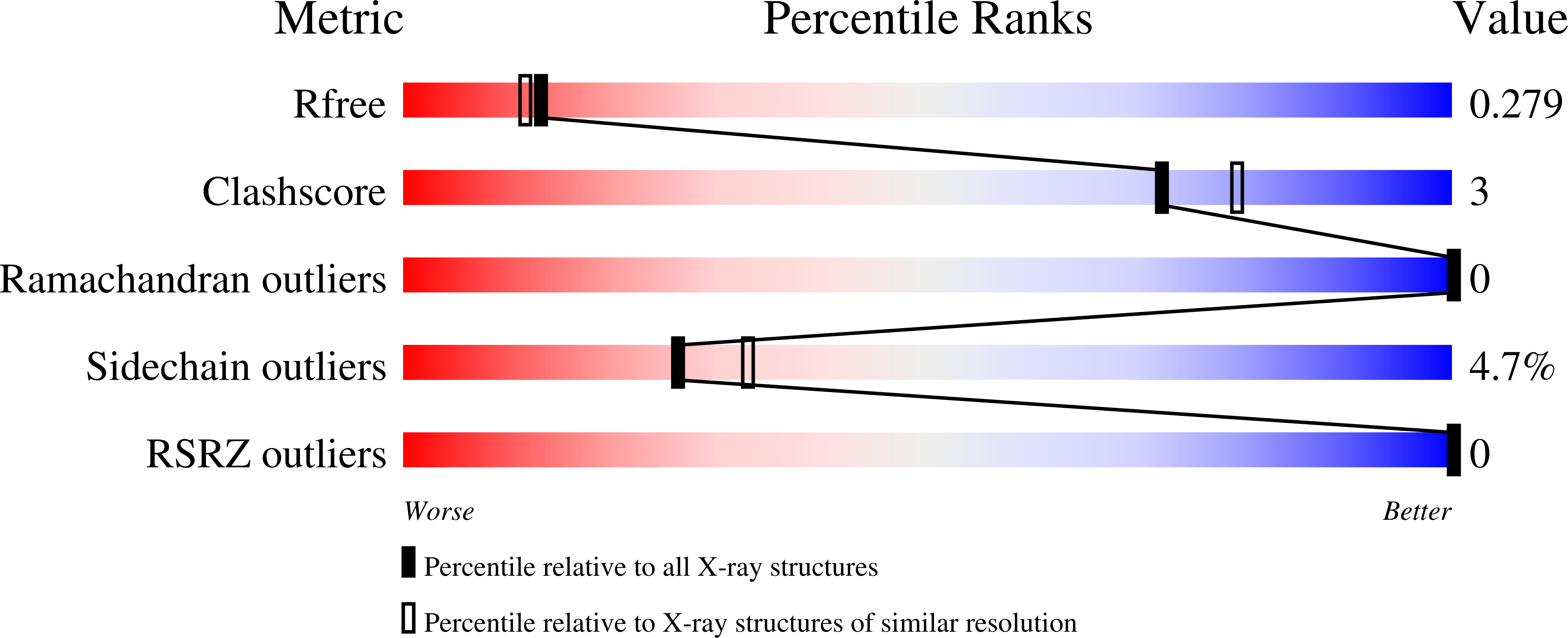
Resolution:
2.20 Å
R-Value Free:
0.28
R-Value Work:
0.21
R-Value Observed:
0.21
Space Group:
P 3 2 1