Deposition Date
2008-04-14
Release Date
2009-04-14
Last Version Date
2023-11-15
Entry Detail
PDB ID:
2ZM9
Keywords:
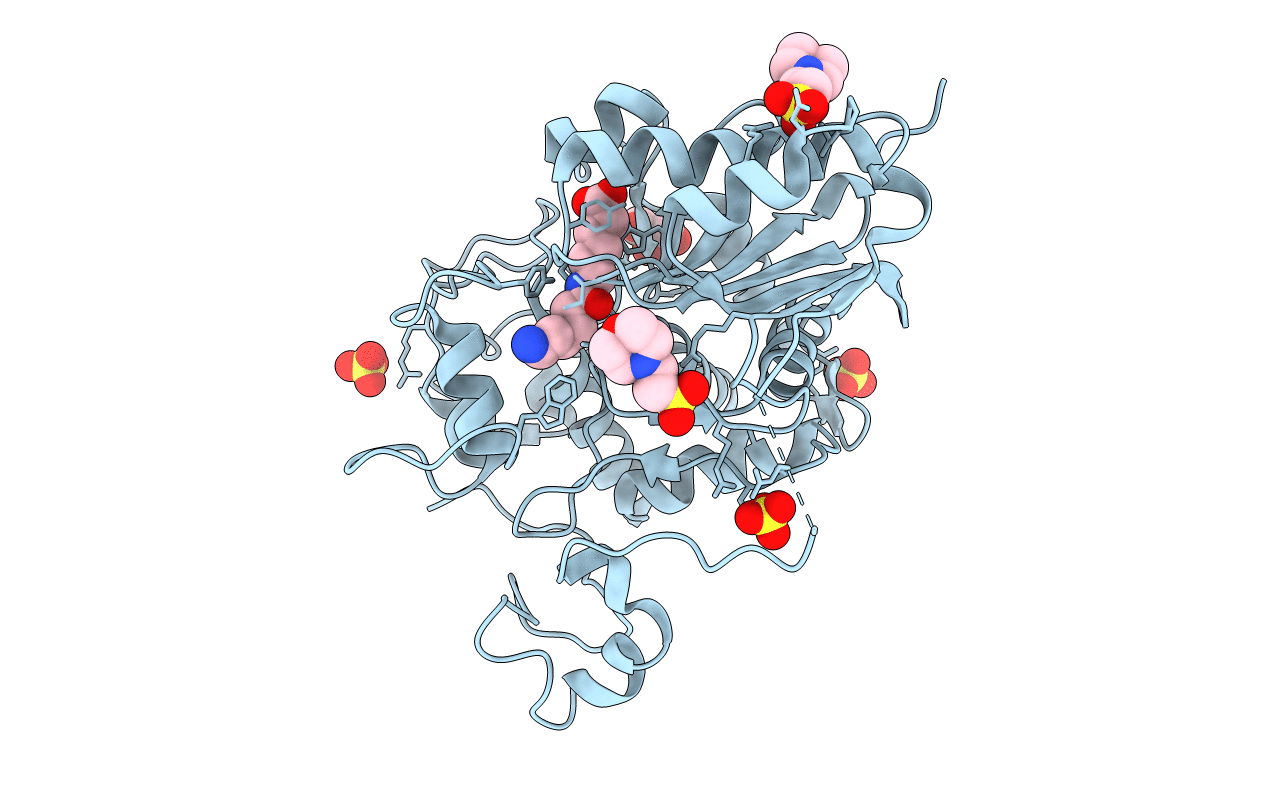
Title:
Structure of 6-Aminohexanoate-dimer Hydrolase, A61V/S112A/A124V/R187S/F264C/G291R/G338A/D370Y mutant (Hyb-S4M94) with Substrate
Biological Source:
Source Organism(s):
Flavobacterium sp. (Taxon ID: 239)
Expression System(s):
Method Details:
Experimental Method:
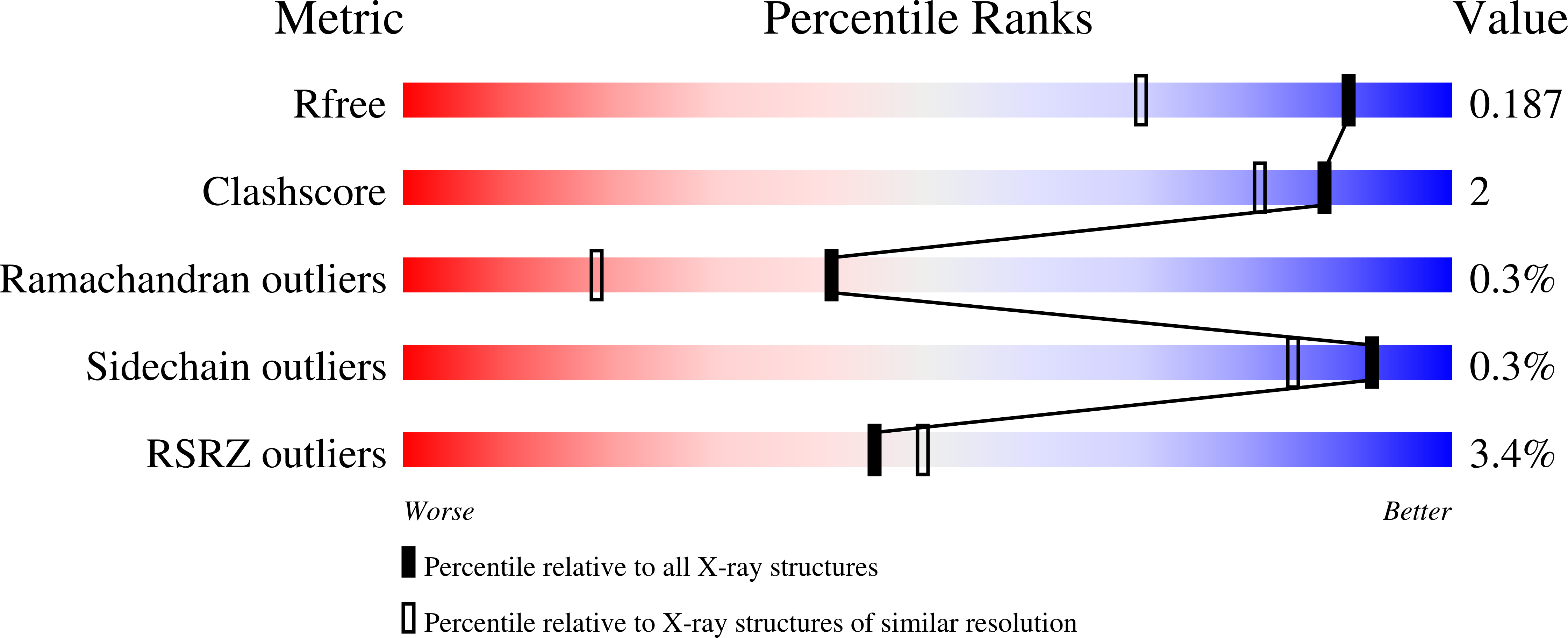
Resolution:
1.50 Å
R-Value Free:
0.19
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
P 32 2 1