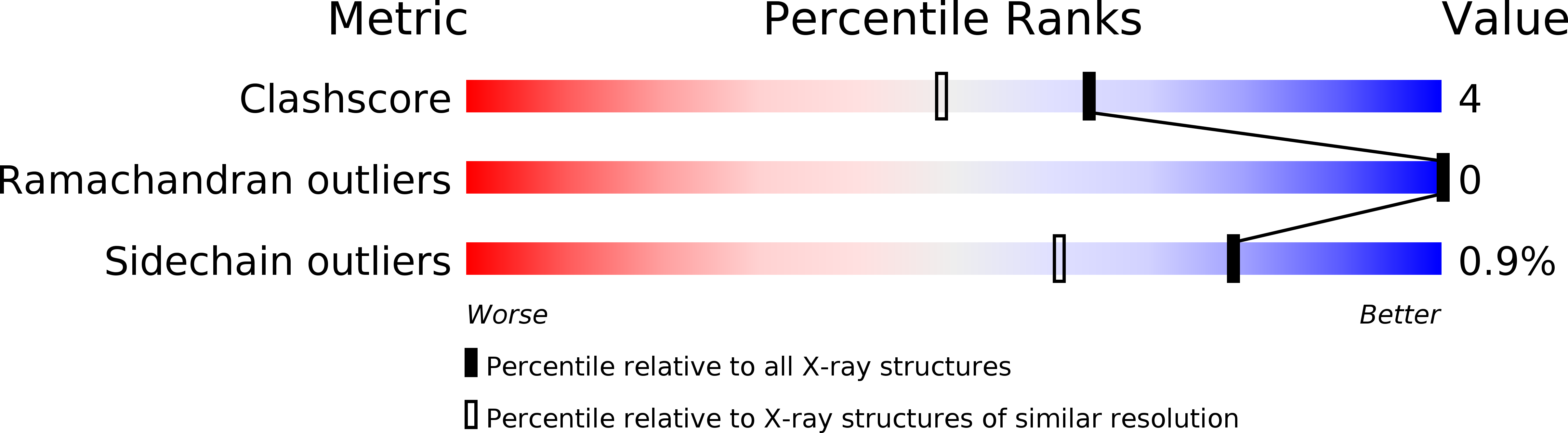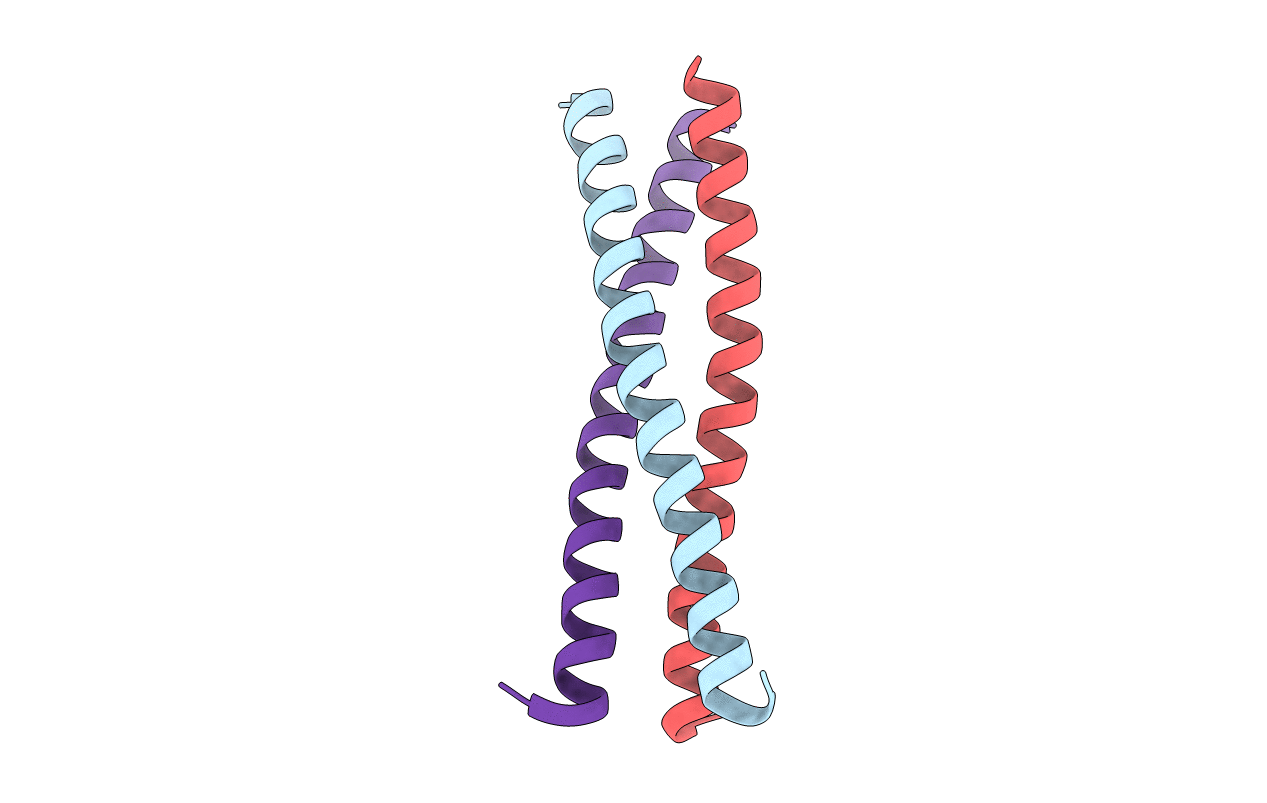
Deposition Date
2007-12-29
Release Date
2008-04-22
Last Version Date
2024-03-13
Entry Detail
PDB ID:
2ZFC
Keywords:
Title:
X-ray crystal structure of an engineered N-terminal HIV-1 GP41 trimer with enhanced stability and potency
Method Details:
Experimental Method:
Resolution:
1.50 Å
R-Value Free:
0.26
R-Value Work:
0.22
Space Group:
H 3